Garnet: Difference between revisions
→Hardness: fix typo |
No edit summary |
||
| Line 1: | Line 1: | ||
A '''nucleophile''' is a [[chemical species]] that donates an [[electron pair]] to an [[electrophile]] to form a [[chemical bond]] in relation to a [[Chemical reaction|reaction]]. All [[molecule]]s or [[ion]]s with a free pair of electrons or at least one [[pi bond]] can act as nucleophiles. Because nucleophiles donate electrons, they are by definition [[Lewis base]]s. | |||
'''Nucleophilic''' describes the affinity of a nucleophile to the nuclei. '''Nucleophilicity''', sometimes referred to as '''nucleophile strength''', refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. | |||
Neutral nucleophilic reactions with solvents such as [[alcohol]]s and water are named [[solvolysis]]. Nucleophiles may take part in [[nucleophilic substitution]], whereby a nucleophile becomes attracted to a full or partial positive charge. | |||
==History== | |||
The terms ''nucleophile'' and ''electrophile'' were introduced by [[Christopher Kelk Ingold]] in 1929,<ref>Ingold, C. K. Recl. TraV. Chim. Pays-Bas '''1929'''</ref> replacing the terms ''anionoid'' and ''cationoid'' proposed earlier by [[A. J. Lapworth]] in 1925.<ref>Lapworth, A. [[Nature (journal)|Nature]] '''1925''', 115, 625</ref> | |||
The word nucleophile is derived from [[atomic nucleus|nucleus]] and the Greek word [[-phil-|φιλος, philos]] for love. | |||
==Properties== | |||
In general, in a row across the periodic table, the more basic the ion (the higher the pK<sub>a</sub> of the conjugate acid) the more reactive it is as a nucleophile. In a given group, [[polarizability]] is more important in the determination of the nucleophilicity: The easier it is to distort the electron cloud around an atom or molecule the more readily it will react; e.g., the [[iodide]] ion (I<sup>−</sup>) is more nucleophilic than the [[fluoride]] ion (F<sup>−</sup>). | |||
===Nucleophilicity=== | |||
Many schemes attempting to quantify relative nucleophilic strength have been devised. The following [[empirical]] data have been obtained by measuring [[reaction rate]]s for a large number of reactions involving a large number of nucleophiles and electrophiles. Nucleophiles displaying the so-called [[alpha effect]] are usually omitted in this type of treatment. | |||
====Swain-Scott equation==== | |||
The first such attempt is found in the Swain–Scott equation<ref>''Quantitative Correlation of Relative Rates. Comparison of Hydroxide Ion with Other Nucleophilic Reagents toward Alkyl Halides, Esters, Epoxides and Acyl Halides'' C. Gardner Swain, Carleton B. Scott [[J. Am. Chem. Soc.]]; '''1953'''; 75(1); 141-147. [http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1953/75/i01/f-pdf/f_ja01097a041.pdf Abstract]</ref><ref>[[Gold Book]] definition (Swain-Scott) [http://www.iupac.org/goldbook/S06201.pdf Link]</ref> derived in 1953: | |||
:<math>\log_{10}\left(\frac{k}{k_0}\right) = sn</math> | |||
This [[free-energy relationship]] relates the [[pseudo first order reaction|pseudo first order]] [[reaction rate constant]] (in water at 25 °C), ''k'', of a reaction, normalized to the reaction rate, ''k''<sub>0</sub>, of a standard reaction with water as the nucleophile, to a nucleophilic constant ''n'' for a given nucleophile and a substrate constant ''s'' that depends on the sensitivity of a substrate to nucleophilic attack (defined as 1 for [[methyl bromide]]). | |||
This treatment results in the following values for typical nucleophilic anions: [[acetate]] 2.7, [[chloride]] 3.0, [[azide]] 4.0, [[hydroxide]] 4.2, [[aniline]] 4.5, [[iodide]] 5.0, and [[thiosulfate]] 6.4. Typical substrate constants are 0.66 for [[tosylate|ethyl tosylate]], 0.77 for [[lactone|β-propiolactone]], 1.00 for [[epoxide|2,3-epoxypropanol]], 0.87 for [[benzyl chloride]], and 1.43 for [[benzoyl chloride]]. | |||
The equation predicts that, in a [[nucleophilic displacement]] on [[benzyl chloride]], the [[azide]] anion reacts 3000 times faster than water. | |||
====Ritchie equation==== | |||
The Ritchie equation, derived in 1972, is another free-energy relationship:<ref>[[Gold Book]] definition (Ritchie) [http://www.iupac.org/goldbook/R05402.pdf Link]</ref><ref>''Nucleophilic reactivities toward cations'' Calvin D. Ritchie Acc. Chem. Res.; '''1972'''; 5(10); 348-354. [http://pubs.acs.org/cgi-bin/abstract.cgi/achre4/1972/5/i10/f-pdf/f_ar50058a005.pdf Abstract] | |||
</ref><ref>''Cation-anion combination reactions. XIII. Correlation of the reactions of nucleophiles with esters'' Calvin D. Ritchie [[J. Am. Chem. Soc.]]; '''1975'''; 97(5); 1170-1179. | |||
[http://pubs.acs.org/cgi-bin/abstract.cgi/jacsat/1975/97/i05/f-pdf/f_ja00838a035.pdf Abstract]</ref> | |||
:<math>\log_{10}\left(\frac{k}{k_0}\right) = N^+</math> | |||
where ''N''<sup>+</sup> is the nucleophile dependent parameter and ''k''<sub>0</sub> the [[reaction rate constant]] for water. In this equation, a substrate-dependent parameter like ''s'' in the Swain–Scott equation is absent. The equation states that two nucleophiles react with the same relative reactivity regardless of the nature of the electrophile, which is in violation of the [[Reactivity–selectivity principle]]. For this reason this equation is also called the ''constant selectivity relationship''. | |||
In the original publication the data were obtained by reactions of selected nucleophiles with selected electrophilic [[carbocation]]s such as [[tropylium]] cations: | |||
or [[diazonium]] cations: | |||
:[[Image:RichieEquationDiazonium.png|400px|Ritchie equation diazonium ion reactions]] | |||
or (not displayed) ions based on [[Malachite green]]. Many other reaction types have since been described. | |||
Typical Ritchie '''N<sup>+</sup>''' values (in [[methanol]]) are: 0.5 for [[methanol]], 5.9 for the [[cyanide]] anion, 7.5 for the [[methoxide]] anion, 8.5 for the [[azide]] anion, and 10.7 for the [[thiophenol]] anion. The values for the relative cation reactivities are -0.4 for the malachite green cation, +2.6 for the [[benzenediazonium cation]], and +4.5 for the [[tropylium cation]]. | |||
====Mayr-Patz equation==== | |||
In the Mayr-Patz equation (1994):<ref>''Scales of Nucleophilicity and Electrophilicity: A System for Ordering Polar Organic and Organometallic Reactions'' [[Angewandte Chemie]] International Edition in English, Vol. 33, No. 9, P. 938-957 {{DOI|10.1002/anie.199409381}}</ref> | |||
<math>\ log(k) = s(N + E)</math> | |||
The [[rate law|second order]] [[reaction rate constant]] '''k''' at 20°C for a reaction is related to a '''nucleophilicity parameter N''', an '''electrophilicity parameter E''', and a '''nucleophile-dependent slope parameter s'''. The constant s is defined as 1 with ''2-methyl-1-pentene'' as the nucleophile. | |||
Many of the constants have been derived from reaction of so-called benzhydrylium ions as the [[electrophile]]s:<ref>''Reference Scales for the Characterization of Cationic Electrophiles and Neutral Nucleophiles''Herbert Mayr, Thorsten Bug, Matthias F. Gotta, Nicole Hering, Bernhard Irrgang, Brigitte Janker, Bernhard Kempf, Robert Loos, Armin R. Ofial, Grigoriy Remennikov, and Holger Schimmel [[J. Am. Chem. Soc.]]; '''2001'''; 123(39) pp 9500 - 9512; (Article) {{DOI|10.1021/ja010890y}}</ref> | |||
[[Image:Benzhydryliumion.png|150px|benzhydrylium ions used in the determination of Mayr-Patz equation]] | |||
and a diverse collection of π-nucleophiles: | |||
:[[Image:MayrNucleophiles.png|300px|Nucleophiles used in the determination of Mayr-Patz equation, X = tetrafluoroborate anion]]. | |||
Typical E values are +6.2 for R = [[chlorine]], +5.90 for R = [[hydrogen]], 0 for R = [[methoxy]] and -7.02 for R = [[dimethylamine]]. | |||
Typical N values with s in parenthesis are -4.47 (1.32) for [[electrophilic aromatic substitution]] to [[toluene]] (1), -0.41 (1.12) for [[electrophilic addition]] to 1-phenyl-2-propene (2), and 0.96 (1) for addition to 2-methyl-1-pentene (3), -0.13 (1.21) for reaction with triphenylallylsilane (4), 3.61 (1.11) for reaction with [[2-methylfuran]] (5), +7.48 (0.89) for reaction with isobutenyltributylstannane (6) and +13.36 (0.81) for reaction with the [[enamine]] 7.<ref>An internet database for reactivity parameters maintained by the Mayr group is available at http://www.cup.uni-muenchen.de/oc/mayr/</ref> | |||
The range of organic reactions also include [[SN2 reaction]]s:<ref name=Mayr2006>''Towards a General Scale of Nucleophilicity?'' Thanh Binh Phan, Martin Breugst, Herbert Mayr, [[Angewandte Chemie International Edition]] | |||
Volume 45, Issue 23 , Pages 3869 - 3874 '''2006''' {{DOI|10.1002/anie.200600542}}</ref> | |||
[[Image:Mayr2006.png|400px|Mayr equation also includes SN2 reactions]] | |||
With E = -9.15 for the ''S-methyldibenzothiophenium ion'', typical nucleophile values N (s) are 15.63 (0.64) for [[piperidine]], 10.49 (0.68) for [[methoxide]], and 5.20 (0.89) for water. In short, nucleophilicities towards sp<sub>2</sub> or sp<sub>3</sub> centers follow the same pattern. | |||
====Unified equation==== | |||
In an effort to unify the above described equations the Mayr equation is rewritten as:<ref name=Mayr2006/> | |||
<math>\log(k) = s_Es_N(N + E)</math> | |||
with s<sub>E</sub> the electrophile-dependent slope parameter and s<sub>N</sub> the nucleophile-dependent slope parameter. This equation can be rewritten in several ways: | |||
* with s<sub>E</sub> = 1 for carbocations this equation is equal to the original Mayr-Patz equation of 1994, | |||
* with s<sub>N</sub> = 0.6 for most n nucleophiles the equation becomes | |||
:<math>\log(k) = 0.6s_EN + 0.6s_EE</math> | |||
:or the original Scott-Swain equation written as: | |||
:<math>\log(k) = \log(k_0) + s_EN</math> | |||
* with s<sub>E</sub> = 1 for carbocations and s<sub>N</sub> = 0.6 the equation becomes: | |||
:<math>\log(k) = 0.6N + 0.6E</math> | |||
:or the original Ritchie equation written as: | |||
:<math>\log(k) - \log(k_0) = N^+</math> | |||
==Types== | |||
Examples of nucleophiles are anions such as Cl<sup>−</sup>, or a compound with a [[lone pair]] of electrons such as NH<sub>3</sub> (ammonia). | |||
In the example below, the [[oxygen]] of the hydroxide ion donates an electron pair to bond with the [[carbon]] at the end of the [[alkyl halide|bromopropane]] molecule. The bond between the carbon and the [[bromine]] then undergoes [[heterolytic fission]], with the bromine atom taking the donated electron and becoming the [[bromide]] ion (Br<sup>−</sup>), because a S<sub>N</sub>2 reaction occurs by backside attack. This means that the hydroxide ion attacks the carbon atom from the other side, exactly opposite the bromine ion. Because of this backside attack, S<sub>N</sub>2 reactions result in a reversal of the [[Molecular configuration|configuration]] of the electrophile. If the electrophile is chiral, it typically maintains its chirality, though the S<sub>N</sub>2 product's configuration is flipped as compared to that of the original electrophile. | |||
[[image:hydrox subst.png|Displacement of bromine by a hydroxide]] | |||
An '''ambident nucleophile''' is one that can attack from two or more places, resulting in two or more products. For example, the [[thiocyanate]] ion (SCN<sup>−</sup>) may attack from either the {{sulfur}} or the {{nitrogen}}. For this reason, the [[SN2 reaction|S<sub>N</sub>2 reaction]] of an alkyl halide with SCN<sup>−</sup> often leads to a mixture of RSCN (an alkyl thiocyanate) and RNCS (an alkyl [[isothiocyanate]]). Similar considerations apply in the [[Kolbe nitrile synthesis]]. | |||
===Carbon=== | |||
Carbon nucleophiles are alkyl metal halides found in the [[Grignard reaction]], [[Blaise reaction]], [[Reformatsky reaction]], and [[Barbier reaction]], [[organolithium reagent]]s, and [[anion]]s of a [[Polymer|terminal]] [[alkyne]]. | |||
Enols are also carbon nucleophiles. The formation of an enol is catalyzed by acid or base. Enols are ambident nucleophiles, but, in general, nucleophilic at the alpha carbon atom. Enols are commonly used in condensation reactions, including the Claisen condensation and the aldol condensation reactions. | |||
===Oxygen=== | |||
Examples of oxygen nucleophiles are [[water]] (H<sub>2</sub>O), [[hydroxide]] anion, [[alcohol]]s, [[alkoxide]] anions, [[hydrogen peroxide]], and [[Carboxylate|carboxylate anions]] | |||
Nuclophilic attack does not take place during intermolecular hydrogen bonding. | |||
===Sulfur=== | |||
Of sulfur nucleophiles, [[hydrogen sulfide]] and its salts, [[thiol]]s (RSH), thiolate anions (RS<sup>−</sup>), anions of thiolcarboxylic acids (RC(O)-S<sup>−</sup>), and anions of dithiocarbonates (RO-C(S)-S<sup>−</sup>) and dithiocarbamates (R<sub>2</sub>N-C(S)-S<sup>−</sup>) are used most often. | |||
In general, sulfur is very nucleophilic because of its large size, which makes it readily polarizable, and its lone pairs of electrons are readily accessible. | |||
===Nitrogen=== | |||
Nitrogen nucleophiles include [[ammonia]], [[azide]], [[amine]]s, and [[nitrite]]s. | |||
== See also == | |||
*[[Electrophile]] | |||
*[[Lewis acids and bases]] | |||
*[[Nucleophilic abstraction]] | |||
*[[Addition to pi ligands]] | |||
==References== | |||
{{reflist}} | |||
[[Category:Physical organic chemistry]] | |||
Revision as of 17:33, 30 January 2014
A nucleophile is a chemical species that donates an electron pair to an electrophile to form a chemical bond in relation to a reaction. All molecules or ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.
Nucleophilic describes the affinity of a nucleophile to the nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms.
Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge.
History
The terms nucleophile and electrophile were introduced by Christopher Kelk Ingold in 1929,[1] replacing the terms anionoid and cationoid proposed earlier by A. J. Lapworth in 1925.[2]
The word nucleophile is derived from nucleus and the Greek word φιλος, philos for love.
Properties
In general, in a row across the periodic table, the more basic the ion (the higher the pKa of the conjugate acid) the more reactive it is as a nucleophile. In a given group, polarizability is more important in the determination of the nucleophilicity: The easier it is to distort the electron cloud around an atom or molecule the more readily it will react; e.g., the iodide ion (I−) is more nucleophilic than the fluoride ion (F−).
Nucleophilicity
Many schemes attempting to quantify relative nucleophilic strength have been devised. The following empirical data have been obtained by measuring reaction rates for a large number of reactions involving a large number of nucleophiles and electrophiles. Nucleophiles displaying the so-called alpha effect are usually omitted in this type of treatment.
Swain-Scott equation
The first such attempt is found in the Swain–Scott equation[3][4] derived in 1953:
This free-energy relationship relates the pseudo first order reaction rate constant (in water at 25 °C), k, of a reaction, normalized to the reaction rate, k0, of a standard reaction with water as the nucleophile, to a nucleophilic constant n for a given nucleophile and a substrate constant s that depends on the sensitivity of a substrate to nucleophilic attack (defined as 1 for methyl bromide).
This treatment results in the following values for typical nucleophilic anions: acetate 2.7, chloride 3.0, azide 4.0, hydroxide 4.2, aniline 4.5, iodide 5.0, and thiosulfate 6.4. Typical substrate constants are 0.66 for ethyl tosylate, 0.77 for β-propiolactone, 1.00 for 2,3-epoxypropanol, 0.87 for benzyl chloride, and 1.43 for benzoyl chloride.
The equation predicts that, in a nucleophilic displacement on benzyl chloride, the azide anion reacts 3000 times faster than water.
Ritchie equation
The Ritchie equation, derived in 1972, is another free-energy relationship:[5][6][7]
where N+ is the nucleophile dependent parameter and k0 the reaction rate constant for water. In this equation, a substrate-dependent parameter like s in the Swain–Scott equation is absent. The equation states that two nucleophiles react with the same relative reactivity regardless of the nature of the electrophile, which is in violation of the Reactivity–selectivity principle. For this reason this equation is also called the constant selectivity relationship.
In the original publication the data were obtained by reactions of selected nucleophiles with selected electrophilic carbocations such as tropylium cations:
or diazonium cations:
or (not displayed) ions based on Malachite green. Many other reaction types have since been described.
Typical Ritchie N+ values (in methanol) are: 0.5 for methanol, 5.9 for the cyanide anion, 7.5 for the methoxide anion, 8.5 for the azide anion, and 10.7 for the thiophenol anion. The values for the relative cation reactivities are -0.4 for the malachite green cation, +2.6 for the benzenediazonium cation, and +4.5 for the tropylium cation.
Mayr-Patz equation
In the Mayr-Patz equation (1994):[8]
The second order reaction rate constant k at 20°C for a reaction is related to a nucleophilicity parameter N, an electrophilicity parameter E, and a nucleophile-dependent slope parameter s. The constant s is defined as 1 with 2-methyl-1-pentene as the nucleophile.
Many of the constants have been derived from reaction of so-called benzhydrylium ions as the electrophiles:[9]
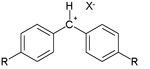 and a diverse collection of π-nucleophiles:
and a diverse collection of π-nucleophiles:
Typical E values are +6.2 for R = chlorine, +5.90 for R = hydrogen, 0 for R = methoxy and -7.02 for R = dimethylamine.
Typical N values with s in parenthesis are -4.47 (1.32) for electrophilic aromatic substitution to toluene (1), -0.41 (1.12) for electrophilic addition to 1-phenyl-2-propene (2), and 0.96 (1) for addition to 2-methyl-1-pentene (3), -0.13 (1.21) for reaction with triphenylallylsilane (4), 3.61 (1.11) for reaction with 2-methylfuran (5), +7.48 (0.89) for reaction with isobutenyltributylstannane (6) and +13.36 (0.81) for reaction with the enamine 7.[10]
The range of organic reactions also include SN2 reactions:[11]
With E = -9.15 for the S-methyldibenzothiophenium ion, typical nucleophile values N (s) are 15.63 (0.64) for piperidine, 10.49 (0.68) for methoxide, and 5.20 (0.89) for water. In short, nucleophilicities towards sp2 or sp3 centers follow the same pattern.
Unified equation
In an effort to unify the above described equations the Mayr equation is rewritten as:[11]
with sE the electrophile-dependent slope parameter and sN the nucleophile-dependent slope parameter. This equation can be rewritten in several ways:
- with sE = 1 for carbocations this equation is equal to the original Mayr-Patz equation of 1994,
- with sN = 0.6 for most n nucleophiles the equation becomes
- with sE = 1 for carbocations and sN = 0.6 the equation becomes:
Types
Examples of nucleophiles are anions such as Cl−, or a compound with a lone pair of electrons such as NH3 (ammonia).
In the example below, the oxygen of the hydroxide ion donates an electron pair to bond with the carbon at the end of the bromopropane molecule. The bond between the carbon and the bromine then undergoes heterolytic fission, with the bromine atom taking the donated electron and becoming the bromide ion (Br−), because a SN2 reaction occurs by backside attack. This means that the hydroxide ion attacks the carbon atom from the other side, exactly opposite the bromine ion. Because of this backside attack, SN2 reactions result in a reversal of the configuration of the electrophile. If the electrophile is chiral, it typically maintains its chirality, though the SN2 product's configuration is flipped as compared to that of the original electrophile.
An ambident nucleophile is one that can attack from two or more places, resulting in two or more products. For example, the thiocyanate ion (SCN−) may attack from either the 56 year old Crop Farmers Danilo Priestly from Dollard-des-Ormeaux, loves railfans, property developers in singapore and kitchen chemistry. Was in recent past paying a visit to Paleochristian and Byzantine Monuments of Thessalonika.
Feel free to visit my blog: Ec new Launch or the We now have developed our capabilities in the commercial real estate for sale estate business to undertake a variety of tasks. These developments vary from residential, business, industrial, resorts and golf courses to retail malls.
Ascott Raffles Place Singapore is a superb property, fantastically designed and outfitted. As I used to be there for two months, one of your employees provided us a really aggressive fee. Additional requests had been met with nice and attentive service. The staff had been genuinely gracious and attentive about our enjoyment of our keep. Ok. Mikaela Braynen The first instrument executed relating to a sale and purchase is liable to advert valorem responsibility (i.e. full responsibility). Subsequent document(s), relating to the identical sale transaction, is liable to nominal responsibility. JLL appointed exclusive agent for the sale of 2, four and 6 Dunlop Road by Expression of Interest. Rare Industrial Improvement inside Pandan Meals Zone space up for sale London (UK) Property Loans United States (US) Property Loans
With differing central financial institution interest rates and Singapore having very low rates of interest similar to the United States (US), banks in Singapore are able to provide SGD and dual currency mortgages which can be competitively lower than onshore banks in host counties. Prospects sometimes enjoy interest financial savings of up to 60%. Following the e-relocation step-by-step information will prevent numerous time and assists you in deciding in your future dwelling in Singapore. This can scale back time you should spend in a short lived home reminiscent of a serviced condo or resort. Centrally positioned in Singapore and within strolling distance to the close by moist market and consuming outlets. HOUSE STRAIGHTFORWARD! We have now greater than 100 Condos for LEASE & for SALE in EAST COAST SG Take a look at SIZZLING PROPERTIES! Terrace Home
Citystate Properties Pte Ltd manages several conservation buildings alongside Club Street. Renovation work on these buildings was accomplished in 1998, restoring these great examples of typical local shophouse structure to their former glory. The rise in the nominal value index pales as compared with the 38.2% worth hike (34% in actual terms) in the course of the 12 months to Q2 2010, a interval which noticed the fastest worth-rises of the recent boom. Measures were launched in October 2012 to restrict mortgage tenure to 35 years, and to lower to 60% the LTV ratio for loans longer than 30 years, or mortgage periods extending beyond age 65. To be a leading homegrown full service international property consultancy firm Mission Gateway to Digital Market Places
Before you resolve to purchase a Home or Flat it's best to ensure that you've sufficientfunds obtainable to complete the acquisition. In case you are financing your buy withyour CPF funds and/or a Mortgage it's best to contact the CPF Board and the Lender bank/financecompany in advance to signal all related software forms and furnish all relevantinformation and paperwork to make sure that the CPF funds and/or Loan would be approvedand the funds will likely be out there. In addition to the acquisition value you will haveto make provision for the stamp charges and Lawyer's charges. Residential Property Act.
The golden handcuffs will cause more of those owners to remain put; many may seek out second mortgages or reverse mortgages to improve their retirement with cash locked up in their homes. Many of those golden handcuff homeowners are in areas with very high-priced actual estate like California, New York, and other areas in excessive demand. Others are throughout the country in excessive-dollar actual estate neighborhoods. There can be fewer properties available, higher costs will outcome, and markets will likely be completely happy.. For this reason, the SN2 reaction of an alkyl halide with SCN− often leads to a mixture of RSCN (an alkyl thiocyanate) and RNCS (an alkyl isothiocyanate). Similar considerations apply in the Kolbe nitrile synthesis.
Carbon
Carbon nucleophiles are alkyl metal halides found in the Grignard reaction, Blaise reaction, Reformatsky reaction, and Barbier reaction, organolithium reagents, and anions of a terminal alkyne.
Enols are also carbon nucleophiles. The formation of an enol is catalyzed by acid or base. Enols are ambident nucleophiles, but, in general, nucleophilic at the alpha carbon atom. Enols are commonly used in condensation reactions, including the Claisen condensation and the aldol condensation reactions.
Oxygen
Examples of oxygen nucleophiles are water (H2O), hydroxide anion, alcohols, alkoxide anions, hydrogen peroxide, and carboxylate anions Nuclophilic attack does not take place during intermolecular hydrogen bonding.
Sulfur
Of sulfur nucleophiles, hydrogen sulfide and its salts, thiols (RSH), thiolate anions (RS−), anions of thiolcarboxylic acids (RC(O)-S−), and anions of dithiocarbonates (RO-C(S)-S−) and dithiocarbamates (R2N-C(S)-S−) are used most often.
In general, sulfur is very nucleophilic because of its large size, which makes it readily polarizable, and its lone pairs of electrons are readily accessible.
Nitrogen
Nitrogen nucleophiles include ammonia, azide, amines, and nitrites.
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- ↑ Ingold, C. K. Recl. TraV. Chim. Pays-Bas 1929
- ↑ Lapworth, A. Nature 1925, 115, 625
- ↑ Quantitative Correlation of Relative Rates. Comparison of Hydroxide Ion with Other Nucleophilic Reagents toward Alkyl Halides, Esters, Epoxides and Acyl Halides C. Gardner Swain, Carleton B. Scott J. Am. Chem. Soc.; 1953; 75(1); 141-147. Abstract
- ↑ Gold Book definition (Swain-Scott) Link
- ↑ Gold Book definition (Ritchie) Link
- ↑ Nucleophilic reactivities toward cations Calvin D. Ritchie Acc. Chem. Res.; 1972; 5(10); 348-354. Abstract
- ↑ Cation-anion combination reactions. XIII. Correlation of the reactions of nucleophiles with esters Calvin D. Ritchie J. Am. Chem. Soc.; 1975; 97(5); 1170-1179. Abstract
- ↑ Scales of Nucleophilicity and Electrophilicity: A System for Ordering Polar Organic and Organometallic Reactions Angewandte Chemie International Edition in English, Vol. 33, No. 9, P. 938-957 Electronic Instrument Positions Staff (Standard ) Cameron from Clarence Creek, usually spends time with hobbies and interests which include knotting, property developers in singapore apartment For sale and boomerangs. Has enrolled in a world contiki journey. Is extremely thrilled specifically about visiting .
- ↑ Reference Scales for the Characterization of Cationic Electrophiles and Neutral NucleophilesHerbert Mayr, Thorsten Bug, Matthias F. Gotta, Nicole Hering, Bernhard Irrgang, Brigitte Janker, Bernhard Kempf, Robert Loos, Armin R. Ofial, Grigoriy Remennikov, and Holger Schimmel J. Am. Chem. Soc.; 2001; 123(39) pp 9500 - 9512; (Article) Electronic Instrument Positions Staff (Standard ) Cameron from Clarence Creek, usually spends time with hobbies and interests which include knotting, property developers in singapore apartment For sale and boomerangs. Has enrolled in a world contiki journey. Is extremely thrilled specifically about visiting .
- ↑ An internet database for reactivity parameters maintained by the Mayr group is available at http://www.cup.uni-muenchen.de/oc/mayr/
- ↑ 11.0 11.1 Towards a General Scale of Nucleophilicity? Thanh Binh Phan, Martin Breugst, Herbert Mayr, Angewandte Chemie International Edition Volume 45, Issue 23 , Pages 3869 - 3874 2006 Electronic Instrument Positions Staff (Standard ) Cameron from Clarence Creek, usually spends time with hobbies and interests which include knotting, property developers in singapore apartment For sale and boomerangs. Has enrolled in a world contiki journey. Is extremely thrilled specifically about visiting .











