Film score: Difference between revisions
en>Adavidb m Disambiguate Alfred Newman to Alfred Newman (composer) using popups; formatting: 18x nbsp-dash, 2x heading-style, 2x whitespace, HTML entity (using Advisor.js) |
en>JonBroxton →Other award nominees and winners: indicate Emmy winners 2000-2013 |
||
| Line 1: | Line 1: | ||
{{More footnotes|date=May 2013}} | |||
[[Image:Spectral lines continous.png|thumb|Continuous spectrum]] | |||
[[Image:Spectral lines emission.png|thumb|[[Emission spectrum|Emission lines]]]] | |||
[[Image:Spectral lines absorption.png|thumb|[[Absorption spectroscopy|Absorption lines]]]] | |||
[[Image:Spectrum of blue sky.svg|thumb|right|320px|Absorption lines for air, under indirect illumination, with the direct light source not visible, so that the gas is not directly between source and detector. Here, [[Fraunhofer lines]] in sunlight and [[Rayleigh scattering]] of this sunlight is the "source." This is the spectrum of a blue sky somewhat close to the horizon, pointing east at around 3 or 4 pm (i.e., Sun in the West) on a clear day.]] | |||
A '''spectral line''' is a dark or bright line in an otherwise uniform and continuous [[spectrum]], resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used as a sort of "atomic fingerprint," as gases emit light at very specific frequencies when exposed to electromagnetic waves, which are displayed in the form of spectral lines. These "fingerprints" can be compared to the previously collected fingerprints of elements, and are thus used to identify the molecular construct of stars and planets which would otherwise be impossible. | |||
== Types of line spectra == | |||
Spectral lines are the result of interaction between a [[quantum system]] (usually [[atom]]s, but sometimes [[molecule]]s or [[atomic nucleus|atomic nuclei]]) and a single [[photon]]. When a photon has about the right amount of energy to allow a change in the energy state of the system (in the case of an atom this is usually an [[electron]] changing [[Electron configuration|orbitals]]), the photon is absorbed. Then it will be spontaneously re-emitted, either in the same frequency as the original or in a cascade, where the sum of the energies of the photons emitted will be equal to the energy of the one absorbed (assuming the system returns to its original state). | |||
Depending on the type of gas, the photon source and what reaches the detector of the instrument, either an '''emission line''' or an '''absorption line''' will be produced. Dark lines in a broad spectrum are produced when a cold gas is between a broad spectrum photon source and the detector. In this case a decrease in the intensity of light in the frequency of the incident photon is seen as the photons are absorbed, then reemitted in random directions, which are mostly in directions different from the original one. This results in an ''absorption line'', since the narrow frequency band of light initially traveling toward the detector, has been effectively scattered in other directions. Absorption lines are produced even during reflection from an illuminated cold gas, as after reflection there is still the opportunity for a selective absorption (and re-scatter) between the point of reflection and the detector. In this case the cold gas need not be directly interposed between light source and the detector, but it is required to not act as a significant independent source of light. By contrast, if the detector sees photons emitted directly from a (hot) glowing gas, then the detector often sees photons emitted in a narrow frequency range by quantum emission processes in atoms in the gas, and this results in an ''emission line.'' | |||
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium capable of letting light pass through it (typically [[gas]] is used). Several elements were discovered by spectroscopic means, such as [[helium]], [[thallium]], and [[cerium]]. Spectral lines also depend on the physical conditions of the gas, so they are widely used to determine the chemical composition of [[star]]s and other celestial bodies that cannot be analyzed by other means, as well as their physical conditions. | |||
Mechanisms other than atom-photon interaction can produce spectral lines. Depending on the exact physical interaction (with molecules, single particles, etc.) the frequency of the involved photons will vary widely, and lines can be observed across the [[electromagnetic spectrum]], from [[radio wave]]s to [[gamma ray]]s. | |||
== Nomenclature == | |||
{{Expand section|date=October 2008}} | |||
Strong spectral lines in the [[visible spectrum|visible]] part of the spectrum often have a unique [[Fraunhofer line]] designation, such as '''K''' for a line at 393.366 nm emerging from singly ionized [[Calcium|Ca]]<sup>+</sup>, though some of the Fraunhofer "lines" are blends of multiple lines from several different [[Chemical species|species]]. In other cases the lines are designated according to the level of [[ion]]ization adding a [[Roman numeral]] to the designation of the [[chemical element]], so that Ca<sup>+</sup> also has the designation '''Ca II'''. Neutral atoms are denoted with the roman number I, singly ionized atoms with II, and so on, so that for example Fe IX (IX, roman 9) represents eight times ionized [[iron]]. More detailed designations usually include the line [[wavelength]] and may include a [[multiplet]] number (for atomic lines) or [[Molecular spectra or band spectra|band designation]] (for molecular lines). Many spectral lines of atomic [[hydrogen]] also have designations within their respective [[Hydrogen spectral series|series]], such as the [[Lyman series]] or [[Balmer series]]. | |||
== Line Broadening and shift == | |||
A spectral line extends over a range of frequencies, not a single frequency (i.e., it has a nonzero [[spectral linewidth|linewidth]]). In addition, its center may be shifted from its nominal central wavelength. There are several reasons for this broadening and shift. These reasons may be divided into two broad categories - broadening due to local conditions and broadening due to extended conditions. Broadening due to local conditions is due to effects which hold in a small region around the emitting element, usually small enough to assure [[local thermodynamic equilibrium]]. Broadening due to extended conditions may result from changes to the spectral distribution of the radiation as it traverses its path to the observer. It also may result from the combining of radiation from a number of regions which are far from each other. | |||
=== Broadening due to local effects === | |||
====Natural broadening==== | |||
The [[uncertainty principle]] relates the lifetime of an excited state (due to the [[Spontaneous emission|spontaneous radiative decay]] or the [[Auger effect|Auger process]]) with the uncertainty of its energy. A short lifetime will have a large energy uncertainty and a broad emission. This broadening effect results in an unshifted [[Lorentzian function|Lorentzian profile]]. The natural broadening can be experimentally altered only to the extent that decay rates can be artificially suppressed or enhanced.<ref>For example, in the following article, decay was suppressed via a microwave cavity, thus reducing the natural broadening: {{cite journal|last=Gabrielse|first =Gerald|coauthors=H. Dehmelt|title=Observation of Inhibited Spontaneous Emission|journal=Physical Review Letters|volume=55|pages=67–70|year=1985|doi=10.1103/PhysRevLett.55.67|pmid=10031682|issue=1|bibcode=1985PhRvL..55...67G}}</ref> | |||
====Thermal Doppler broadening==== | |||
{{Main|Doppler broadening}} | |||
The atoms in a gas which are emitting radiation will have a distribution of velocities. Each photon emitted will be "red"- or "blue"-shifted by the [[Doppler effect]] depending on the velocity of the atom relative to the observer. The higher the temperature of the gas, the wider the distribution of velocities in the gas. Since the spectral line is a combination of all of the emitted radiation, the higher the temperature of the gas, the broader will be the spectral line emitted from that gas. This broadening effect is described by a [[Gaussian function|Gaussian profile]] and there is no associated shift. | |||
====Pressure broadening==== | |||
The presence of nearby particles will affect the radiation emitted by an individual particle. There are two limiting cases by which this occurs: | |||
*''Impact pressure broadening'' or ''collisional broadening'': The collision of other particles with the emitting particle interrupts the emission process, and by shortening the characteristic time for the process, increases the uncertainty in the energy emitted (as occurs in natural broadening)[http://www.fas.harvard.edu/~scdiroff/lds/QuantumRelativity/CollisionalBroadening/CollisionalBroadening.html]. The duration of the collision is much shorter than the lifetime of the emission process. This effect depends on both the [[density]] and the [[temperature]] of the gas. The broadening effect is described by a [[Lorentzian function|Lorentzian profile]] and there may be an associated shift. | |||
*''Quasistatic pressure broadening'': The presence of other particles shifts the energy levels in the emitting particle, thereby altering the frequency of the emitted radiation. The duration of the influence is much longer than the lifetime of the emission process. This effect depends on the [[density]] of the gas, but is rather insensitive to [[temperature]]. The form of the line profile is determined by the functional form of the perturbing force with respect to distance from the perturbing particle. There may also be a shift in the line center. A [[stable distribution]] is a general expression for the lineshape resulting from quasistatic pressure broadening <ref>{{cite journal | |||
| first = G. | last = Peach | year = 1981 | |||
| title = Theory of the pressure broadening and shift of spectral lines | |||
| journal = Advances in Physics | volume = 30 | issue = 3 | pages = 367–474 | |||
| url = http://journalsonline.tandf.co.uk/openurl.asp?genre=article&eissn=1460-6976&volume=30&issue=3&spage=367 | |||
| doi = 10.1080/00018738100101467 | |||
|bibcode = 1981AdPhy..30..367P }}</ref> | |||
Pressure broadening may also be classified by the nature of the perturbing force as follows: | |||
*''Linear Stark broadening'' occurs via the [[linear Stark effect]] which results from the interaction of an emitter with an electric field, which causes a shift in energy which is linear in the field strength. <math>(\Delta E \sim 1/r^2)</math> | |||
*''Resonance broadening'' occurs when the perturbing particle is of the same type as the emitting particle, which introduces the possibility of an energy exchange process. <math>(\Delta E \sim 1/r^3)</math> | |||
*''Quadratic Stark broadening'' occurs via the [[quadratic Stark effect]] which results from the interaction of an emitter with an electric field, which causes a shift in energy which is quadratic in the field strength. <math>(\Delta E \sim 1/r^4)</math> | |||
*''Van der Waals broadening'' occurs when the emitting particle is being perturbed by [[van der Waals force]]s. For the quasistatic case, a [[Lévy distribution|van der Waals profile]]<ref group="note">"van der Waals profile" appears as lowercase in almost all sources, such as: ''Statistical mechanics of the liquid surface'' by Clive Anthony Croxton, 1980, A Wiley-Interscience publication, ISBN 0-471-27663-4, ISBN 978-0-471-27663-0, [http://books.google.it/books?id=Wve2AAAAIAAJ&q=%22Van+der+Waals+profile%22&dq=%22Van+der+Waals+profile%22&hl=en]; and in ''Journal of technical physics'', Volume 36, by Instytut Podstawowych Problemów Techniki (Polska Akademia Nauk), publisher: Państwowe Wydawn. Naukowe., 1995, [http://books.google.it/books?id=2XpVAAAAMAAJ&q=%22Van+der+Waals+profile%22&dq=%22Van+der+Waals+profile%22&hl=en]<!-- and many more --></ref> is often useful in describing the profile. The energy shift as a function of distance is given in the wings by e.g. the [[Lennard-Jones potential]]. <math>(\Delta E \sim 1/r^6)</math> | |||
====Inhomogeneous broadening==== | |||
''[[Inhomogeneous broadening]]'' is a general term for broadening because some emitting particles are in a different local environment than others, and therefore emit at a different frequency. This term is used especially for solids, where surfaces, grain boundaries, and stoichiometry variations can create a variety of local environments for a given atom to occupy. In liquids, the effects of inhomogeneous broadening is sometimes reduced by a process called ''[[motional narrowing]]''. | |||
=== Broadening due to non-local effects === | |||
Certain types of broadening are the result of conditions over a large region of space rather than simply upon conditions that are local to the emitting particle. | |||
====Opacity broadening==== | |||
Electromagnetic radiation emitted at a particular point in space can be absorbed as it travels through space. This absorption depends on wavelength. The line is broadened because photons at the line wings have a smaller reabsorption probability than photons at the line center. Indeed, the absorption near line center may be so great as to cause a '''self reversal''' in which the intensity at the center of the line is less than in the wings. This process is also sometimes called '''self-absorption'''. | |||
====Macroscopic Doppler broadening==== | |||
Radiation emitted by a moving source is subject to [[Doppler shift]] due to a finite line-of-sight velocity projection. If different parts of the emitting body have different velocities (along the line of sight), the resulting line will be broadened, with the line width proportional to the width of the velocity distribution. For example, radiation emitted from a distant rotating body, such as a [[star]], will be broadened due to the line-of-sight variations in velocity on opposite sides of the star. The greater the rate of rotation, the broader the line. Another example is an imploding [[Plasma (physics)|plasma]] shell in a [[Z-pinch]]. | |||
=== Combined effects === | |||
Each of these mechanisms can act in isolation or in combination with others. Assuming each effect is independent, the observed line profile is a convolution of the line profiles of each mechanism. For example, a combination of the thermal Doppler broadening and the impact pressure broadening yields a [[Voigt profile]]. | |||
However, the different line broadening mechanisms are not always independent. For example, the collisional effects and the motional Doppler shifts can act in a coherent manner, resulting under some conditions even in a collisional ''narrowing'', known as the [[Dicke effect]]. | |||
== See also == | |||
* [[Absorption spectrum]] | |||
* [[Atomic spectral line]] | |||
* [[Bohr model]] | |||
* [[Electron configuration]] | |||
* [[Emission spectrum]] | |||
* [[Spectroscopy]] | |||
* [[Fraunhofer line]] | |||
* [[Hydrogen line]] | |||
* [[Splatalogue]] | |||
==Notes== | |||
{{Reflist|group="note"}} | |||
== References == | |||
{{reflist}} | |||
==Further reading== | |||
* {{cite book | |||
| first = Hans R. | last = Griem | year = 1997 | |||
| title = Principles of Plasmas Spectroscopy | |||
| publisher = University Press | location = Cambridge | isbn = 0-521-45504-9 }} | |||
* {{cite book | |||
| first = Hans R. | last = Griem | year = 1974 | |||
| title = Spectral Line Broadening by Plasmas | |||
| publisher = Academic Press | location = New York | isbn = 0-12-302850-7 }} | |||
* {{cite book | |||
| first = Hans R. | last = Griem | year = 1964 | |||
| title = Plasma Spectroscopy | |||
| publisher = McGraw-Hill book Company | location = New York }} | |||
{{DEFAULTSORT:Spectral Line}} | |||
[[Category:Spectroscopy]] | |||
[[Category:Emission spectroscopy]] | |||
Revision as of 23:56, 31 January 2014



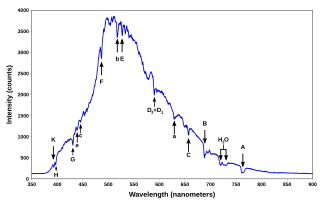
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used as a sort of "atomic fingerprint," as gases emit light at very specific frequencies when exposed to electromagnetic waves, which are displayed in the form of spectral lines. These "fingerprints" can be compared to the previously collected fingerprints of elements, and are thus used to identify the molecular construct of stars and planets which would otherwise be impossible.
Types of line spectra
Spectral lines are the result of interaction between a quantum system (usually atoms, but sometimes molecules or atomic nuclei) and a single photon. When a photon has about the right amount of energy to allow a change in the energy state of the system (in the case of an atom this is usually an electron changing orbitals), the photon is absorbed. Then it will be spontaneously re-emitted, either in the same frequency as the original or in a cascade, where the sum of the energies of the photons emitted will be equal to the energy of the one absorbed (assuming the system returns to its original state).
Depending on the type of gas, the photon source and what reaches the detector of the instrument, either an emission line or an absorption line will be produced. Dark lines in a broad spectrum are produced when a cold gas is between a broad spectrum photon source and the detector. In this case a decrease in the intensity of light in the frequency of the incident photon is seen as the photons are absorbed, then reemitted in random directions, which are mostly in directions different from the original one. This results in an absorption line, since the narrow frequency band of light initially traveling toward the detector, has been effectively scattered in other directions. Absorption lines are produced even during reflection from an illuminated cold gas, as after reflection there is still the opportunity for a selective absorption (and re-scatter) between the point of reflection and the detector. In this case the cold gas need not be directly interposed between light source and the detector, but it is required to not act as a significant independent source of light. By contrast, if the detector sees photons emitted directly from a (hot) glowing gas, then the detector often sees photons emitted in a narrow frequency range by quantum emission processes in atoms in the gas, and this results in an emission line.
Spectral lines are highly atom-specific, and can be used to identify the chemical composition of any medium capable of letting light pass through it (typically gas is used). Several elements were discovered by spectroscopic means, such as helium, thallium, and cerium. Spectral lines also depend on the physical conditions of the gas, so they are widely used to determine the chemical composition of stars and other celestial bodies that cannot be analyzed by other means, as well as their physical conditions.
Mechanisms other than atom-photon interaction can produce spectral lines. Depending on the exact physical interaction (with molecules, single particles, etc.) the frequency of the involved photons will vary widely, and lines can be observed across the electromagnetic spectrum, from radio waves to gamma rays.
Nomenclature
Template:Expand section Strong spectral lines in the visible part of the spectrum often have a unique Fraunhofer line designation, such as K for a line at 393.366 nm emerging from singly ionized Ca+, though some of the Fraunhofer "lines" are blends of multiple lines from several different species. In other cases the lines are designated according to the level of ionization adding a Roman numeral to the designation of the chemical element, so that Ca+ also has the designation Ca II. Neutral atoms are denoted with the roman number I, singly ionized atoms with II, and so on, so that for example Fe IX (IX, roman 9) represents eight times ionized iron. More detailed designations usually include the line wavelength and may include a multiplet number (for atomic lines) or band designation (for molecular lines). Many spectral lines of atomic hydrogen also have designations within their respective series, such as the Lyman series or Balmer series.
Line Broadening and shift
A spectral line extends over a range of frequencies, not a single frequency (i.e., it has a nonzero linewidth). In addition, its center may be shifted from its nominal central wavelength. There are several reasons for this broadening and shift. These reasons may be divided into two broad categories - broadening due to local conditions and broadening due to extended conditions. Broadening due to local conditions is due to effects which hold in a small region around the emitting element, usually small enough to assure local thermodynamic equilibrium. Broadening due to extended conditions may result from changes to the spectral distribution of the radiation as it traverses its path to the observer. It also may result from the combining of radiation from a number of regions which are far from each other.
Broadening due to local effects
Natural broadening
The uncertainty principle relates the lifetime of an excited state (due to the spontaneous radiative decay or the Auger process) with the uncertainty of its energy. A short lifetime will have a large energy uncertainty and a broad emission. This broadening effect results in an unshifted Lorentzian profile. The natural broadening can be experimentally altered only to the extent that decay rates can be artificially suppressed or enhanced.[1]
Thermal Doppler broadening
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. The atoms in a gas which are emitting radiation will have a distribution of velocities. Each photon emitted will be "red"- or "blue"-shifted by the Doppler effect depending on the velocity of the atom relative to the observer. The higher the temperature of the gas, the wider the distribution of velocities in the gas. Since the spectral line is a combination of all of the emitted radiation, the higher the temperature of the gas, the broader will be the spectral line emitted from that gas. This broadening effect is described by a Gaussian profile and there is no associated shift.
Pressure broadening
The presence of nearby particles will affect the radiation emitted by an individual particle. There are two limiting cases by which this occurs:
- Impact pressure broadening or collisional broadening: The collision of other particles with the emitting particle interrupts the emission process, and by shortening the characteristic time for the process, increases the uncertainty in the energy emitted (as occurs in natural broadening)[1]. The duration of the collision is much shorter than the lifetime of the emission process. This effect depends on both the density and the temperature of the gas. The broadening effect is described by a Lorentzian profile and there may be an associated shift.
- Quasistatic pressure broadening: The presence of other particles shifts the energy levels in the emitting particle, thereby altering the frequency of the emitted radiation. The duration of the influence is much longer than the lifetime of the emission process. This effect depends on the density of the gas, but is rather insensitive to temperature. The form of the line profile is determined by the functional form of the perturbing force with respect to distance from the perturbing particle. There may also be a shift in the line center. A stable distribution is a general expression for the lineshape resulting from quasistatic pressure broadening [2]
Pressure broadening may also be classified by the nature of the perturbing force as follows:
- Linear Stark broadening occurs via the linear Stark effect which results from the interaction of an emitter with an electric field, which causes a shift in energy which is linear in the field strength.
- Resonance broadening occurs when the perturbing particle is of the same type as the emitting particle, which introduces the possibility of an energy exchange process.
- Quadratic Stark broadening occurs via the quadratic Stark effect which results from the interaction of an emitter with an electric field, which causes a shift in energy which is quadratic in the field strength.
- Van der Waals broadening occurs when the emitting particle is being perturbed by van der Waals forces. For the quasistatic case, a van der Waals profile[note 1] is often useful in describing the profile. The energy shift as a function of distance is given in the wings by e.g. the Lennard-Jones potential.
Inhomogeneous broadening
Inhomogeneous broadening is a general term for broadening because some emitting particles are in a different local environment than others, and therefore emit at a different frequency. This term is used especially for solids, where surfaces, grain boundaries, and stoichiometry variations can create a variety of local environments for a given atom to occupy. In liquids, the effects of inhomogeneous broadening is sometimes reduced by a process called motional narrowing.
Broadening due to non-local effects
Certain types of broadening are the result of conditions over a large region of space rather than simply upon conditions that are local to the emitting particle.
Opacity broadening
Electromagnetic radiation emitted at a particular point in space can be absorbed as it travels through space. This absorption depends on wavelength. The line is broadened because photons at the line wings have a smaller reabsorption probability than photons at the line center. Indeed, the absorption near line center may be so great as to cause a self reversal in which the intensity at the center of the line is less than in the wings. This process is also sometimes called self-absorption.
Macroscopic Doppler broadening
Radiation emitted by a moving source is subject to Doppler shift due to a finite line-of-sight velocity projection. If different parts of the emitting body have different velocities (along the line of sight), the resulting line will be broadened, with the line width proportional to the width of the velocity distribution. For example, radiation emitted from a distant rotating body, such as a star, will be broadened due to the line-of-sight variations in velocity on opposite sides of the star. The greater the rate of rotation, the broader the line. Another example is an imploding plasma shell in a Z-pinch.
Combined effects
Each of these mechanisms can act in isolation or in combination with others. Assuming each effect is independent, the observed line profile is a convolution of the line profiles of each mechanism. For example, a combination of the thermal Doppler broadening and the impact pressure broadening yields a Voigt profile.
However, the different line broadening mechanisms are not always independent. For example, the collisional effects and the motional Doppler shifts can act in a coherent manner, resulting under some conditions even in a collisional narrowing, known as the Dicke effect.
See also
- Absorption spectrum
- Atomic spectral line
- Bohr model
- Electron configuration
- Emission spectrum
- Spectroscopy
- Fraunhofer line
- Hydrogen line
- Splatalogue
Notes
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
Further reading
- 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534
- ↑ For example, in the following article, decay was suppressed via a microwave cavity, thus reducing the natural broadening: One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang
Cite error: <ref> tags exist for a group named "note", but no corresponding <references group="note"/> tag was found



