Singular solution: Difference between revisions
Salix alba (talk | contribs) →Bibliography: fix sprinter eom link |
en>Yobot m Link equal to linktext using AWB (9585) |
||
| Line 1: | Line 1: | ||
The '''density of air''', ρ (Greek: rho) (air density), is the mass per unit volume of [[Atmosphere of Earth|Earth's atmosphere]], and is a useful value in [[aeronautics]] and other sciences. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in temperature or [[humidity]]. At [[sea level]] and at 15 °C, air has a density of approximately 1.225 kg/m<sup>3</sup> (0.0023769 slugs/ft<sup>3</sup>, 0.001225 g/cm<sup>3</sup>) according to ISA ([[International Standard Atmosphere]]). | |||
== Temperature and pressure == | |||
The density of dry air can be calculated using the [[ideal gas law]], expressed as a function of [[thermodynamic temperature|temperature]] and pressure: | |||
:<math> | |||
\rho = \frac{p}{R_{\rm specific} T} | |||
</math> | |||
where ''ρ'' is the air density, ''p'' is absolute [[pressure]], ''R<sub>specific</sub>'' is the [[specific gas constant]] for dry air, and ''T'' is absolute temperature (K). | |||
The specific gas constant for dry air is 287.058 J/(kg·K) in [[International System of Units|SI]] units, and 53.35 ([[Foot (length)|ft]]·[[Pound-force|lb<sub>f</sub>]])/([[Pound (mass)|lb<sub>m</sub>]]·[[Rankine scale|R]]) in [[United States customary units|United States customary]] and [[Imperial units]]. This quantity may vary slightly depending on the molecular composition of air at a particular location. | |||
Therefore: | |||
*At [[International Union of Pure and Applied Chemistry|IUPAC]] [[standard temperature and pressure]] (0 [[Celsius|°C]] and 100 k[[Pascal (unit)|Pa]]), dry air has a density of 1.2754 [[Kilogram|kg]]/m<sup>3</sup>. | |||
*At 20 °C and 101.325 kPa, dry air has a density of 1.2041 kg/m<sup>3</sup>. | |||
*At 70 [[Fahrenheit|°F]] and 14.696 [[Pounds per square inch|psi]], dry air has a density of 0.074887[[Pound (mass)|lb<sub>m</sub>]]/[[Cubic foot|ft<sup>3</sup>]]. | |||
The following table illustrates the air density–temperature relationship at 1 atm or 101.325 kPa: | |||
{{Temperature_effect}} | |||
=== Water vapor === | |||
The addition of [[water vapor]] to air (making the air humid) reduces the density of the air, which may at first appear counter-intuitive. | |||
This occurs because the molecular mass of water (18 g/mol) is less than the molecular mass of dry air (around 29 g/mol). For any gas, at a given temperature and pressure, the number of molecules present is constant for a particular volume (see [[Avogadro's Law]]). So when water molecules (vapor) are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing. Hence the mass per unit volume of the gas (its density) decreases. | |||
The density of humid air may be calculated as a mixture of [[ideal gas]]es. In this case, the [[partial pressure]] of [[water vapor]] is known as the [[vapor pressure]]. Using this method, error in the density calculation is less than 0.2% in the range of −10 °C to 50 °C. | |||
The density of humid air is found by: | |||
:<math> | |||
\rho_{\,\mathrm{humid~air}} = \frac{p_{d}}{R_{d} T} + \frac{p_{v}}{R_{v} T} = \frac{p_{d}M_{d}+p_{v}M_{v}}{R T} \, | |||
</math><ref name=wahiduddin_01>[http://wahiduddin.net/calc/density_altitude.htm Equations - Air Density and Density Altitude<!-- Bot generated title -->]</ref> | |||
where: | |||
:<math>\rho_{\,\mathrm{humid~air}} =</math> Density of the humid air (kg/m³) | |||
:<math>p_{d} =</math> Partial pressure of dry air (Pa) | |||
:<math>R_{d} =</math> Specific gas constant for dry air, 287.058 J/(kg·K) | |||
:<math>T =</math> Temperature (K) | |||
:<math>p_{v} =</math> Pressure of water vapor (Pa) | |||
:<math>R_{v} =</math> Specific gas constant for water vapor, 461.495 J/(kg·K) | |||
:<math>M_{d} =</math> Molar mass of dry air, 0.028964 (kg/mol) | |||
:<math>M_{v} =</math> Molar mass of water vapor, 0.018016 (kg/mol) | |||
:<math>R =</math> [[Gas_constant|Universal gas constant]], 8.314 J/(K·mol) | |||
The vapor pressure of water may be calculated from the [[saturation vapor pressure]] and [[relative humidity]]. It is found by: | |||
:<math> | |||
p_{v} = \phi p_{\mathrm{sat}} \, | |||
</math> | |||
Where: | |||
:<math>p_{v} =</math> Vapor pressure of water | |||
:<math>\phi =</math> Relative humidity | |||
:<math>p_{\mathrm{sat}} =</math> Saturation vapor pressure | |||
The saturation [[vapour pressure of water | vapor pressure of water]] at any given temperature is the vapor pressure when relative humidity is 100%. One formula <ref name=wahiduddin_02>[http://wahiduddin.net/calc/density_algorithms.htm Algorithms - Schlatter and Baker<!-- Bot generated title -->]</ref> used to find the saturation vapor pressure is: | |||
:<math>p_{\mathrm{sat}} = 6.1078 \times 10^{\frac{7.5 T}{T+237.3}} </math> | |||
where T is in degrees C. | |||
Note: | |||
*This will give a result in [[Pascal_(unit)|hPa]] (100 [[Pascal_(unit)|Pa]], equivalent to the older unit [[millibar]], 1 mbar = 0.001 [[Bar_(unit)|bar]] = 0.1 kPa) | |||
*<math>p_{d}</math> is found considering [[partial pressure]], resulting in: | |||
:<math> | |||
p_{d} = p-p_{v} \, | |||
</math> | |||
Where ''p'' simply denotes the observed [[absolute pressure]]. | |||
=== Altitude === | |||
[[Image:StandardAtmosphere.png|thumb|upright=2.0|Standard Atmosphere: ''p''<sub>0</sub>=101.325 [[Pascal (unit)|kPa]], ''T<sub>0</sub>''=288.15 [[Kelvin|K]], ''ρ<sub>0</sub>''=1.225 [[kg/m³]]]] | |||
To calculate the density of air as a function of altitude, one requires additional parameters. They are listed below, along with their values according to the [[International Standard Atmosphere]], using the [[Gas constant|universal gas constant]] instead of the specific one: | |||
*sea level standard atmospheric pressure ''p''<sub>0</sub> = 101.325 k[[Pascal (unit)|Pa]] | |||
*sea level standard temperature ''T<sub>0</sub>'' = 288.15 [[Kelvin|K]] | |||
*Earth-surface gravitational acceleration ''g'' = 9.80665 m/s<sup>2</sup>. | |||
*[[adiabatic lapse rate|temperature lapse rate]] ''L'' = 0.0065 [[Kelvin|K]]/m | |||
*ideal (universal) gas constant ''R'' = 8.31447 J/([[Mole (unit)|mol]]·K) | |||
*[[molar mass]] of dry air ''M'' = 0.0289644 kg/mol | |||
Temperature at altitude ''h'' meters above sea level is approximated by the following formula (only valid inside the [[troposphere]]): | |||
:<math> | |||
T = T_0 - L h \, | |||
</math> | |||
The pressure at altitude ''h'' is given by: | |||
:<math>p = p_0 \left(1 - \frac{L h}{T_0} \right)^\frac{g M}{R L}</math> | |||
Density can then be calculated according to a molar form of the [[ideal gas law]]: | |||
:<math> | |||
\rho = \frac{p M}{R T} \, | |||
</math> | |||
where ''M'' is [[molar mass]], ''R'' is the [[ideal gas constant]], and ''T'' is [[absolute temperature]]. Note that p must be in Pa and not the kPa above. | |||
== See also == | |||
*[[International Standard Atmosphere]] | |||
*[[U.S. Standard Atmosphere]] | |||
*[[NRLMSISE-00]] | |||
== References == | |||
<references/> | |||
== External links == | |||
*[http://www.sengpielaudio.com/ConvDensi.htm Conversions of density units ρ] | |||
*[http://wahiduddin.net/calc/density_altitude.htm Air density and density altitude calculations] | |||
*[http://www.enggcyclopedia.com/calculators/physical-properties/air-density-calculator/ Air Density Calculator] | |||
*[http://www.wolfdynamics.com/tools/atmospheric-pressure-calculator.html Atmospheric Pressure Calculator] | |||
[[Category:Atmospheric thermodynamics]] | |||
[[Category:Density]] | |||
Latest revision as of 11:10, 7 November 2013
The density of air, ρ (Greek: rho) (air density), is the mass per unit volume of Earth's atmosphere, and is a useful value in aeronautics and other sciences. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in temperature or humidity. At sea level and at 15 °C, air has a density of approximately 1.225 kg/m3 (0.0023769 slugs/ft3, 0.001225 g/cm3) according to ISA (International Standard Atmosphere).
Temperature and pressure
The density of dry air can be calculated using the ideal gas law, expressed as a function of temperature and pressure:
where ρ is the air density, p is absolute pressure, Rspecific is the specific gas constant for dry air, and T is absolute temperature (K).
The specific gas constant for dry air is 287.058 J/(kg·K) in SI units, and 53.35 (ft·lbf)/(lbm·R) in United States customary and Imperial units. This quantity may vary slightly depending on the molecular composition of air at a particular location.
Therefore:
- At IUPAC standard temperature and pressure (0 °C and 100 kPa), dry air has a density of 1.2754 kg/m3.
- At 20 °C and 101.325 kPa, dry air has a density of 1.2041 kg/m3.
- At 70 °F and 14.696 psi, dry air has a density of 0.074887lbm/ft3.
The following table illustrates the air density–temperature relationship at 1 atm or 101.325 kPa:
They're always ready to help, and they're always making changes to the site to make sure you won't have troubles in the first place. The next step is to visit your Word - Press blog dashboard. These templates are professionally designed and are also Adsense ready. Word - Press also provides protection against spamming, as security is a measure issue. After activating, you will find their website link and get the activation code from their website.
Any business enterprise that is certainly worth its name should really shell out a good deal in making sure that they have the most effective website that provides related info to its prospect. But as expected the level of support you get with them can be hit or miss based on the developer's free time and desire. Well Managed Administration The Word - Press can easily absorb the high numbers of traffic by controlling the server load to make sure that the site works properly. Apart from these, you are also required to give some backlinks on other sites as well. You can also get a free keyword tool that is to determine how strong other competing sites are and number of the searches on the most popular search sites.
Digital photography is a innovative effort, if you removethe stress to catch every position and viewpoint of a place, you free yourself up to be more innovative and your outcomes will be much better. The following piece of content is meant to make your choice easier and reassure you that the decision to go ahead with this conversion is requited with rich benefits:. If you adored this information and you would such as to get additional information regarding wordpress backup kindly check out the web site. Possibly the most downloaded Word - Press plugin, the Google XML Sitemaps plugin but not only automatically creates a site map linking to everyone your pages and posts, it also notifies Google, Bing, Yahoo, and Ask. Nonetheless, with stylish Facebook themes obtainable on the Globe Broad Internet, half of your enterprise is done previously. Purchase these from our site, or bring your own, it doesn't matter, we will still give you free installation and configuration.
Digg Digg Social Sharing - This plugin that is accountable for the floating social icon located at the left aspect corner of just about every submit. * Robust CRM to control and connect with your subscribers. When we talk about functional suitability, Word - Press proves itself as one of the strongest contestant among its other rivals. If you are looking for Hire Wordpress Developer then just get in touch with him. Where from they are coming, which types of posts are getting top traffic and many more.
This advice is critical because you don't want to waste too expensive time establishing your Word - Press blog the exact method. In fact portfolio Word - Press themes is a smooth and attractive but considerably flawed Word - Press theme in creating simpler to the photographers or designers to develop a specific internet site showcasing their most current perform since it appear modern-day and has fantastic typography and large photographs which would develop an attractive wanting portfolio internet site. It can be concluded that white label SEO comprise of a third party who resells a contract involving IT expert or consultant, SEO professional and end user. Web developers and newbies alike will have the ability to extend your web site and fit other incredible functions with out having to spend more. However, if you're just starting out your blog site or business site, you can still search for an ideal theme for it without breaking your bank account.
Water vapor
The addition of water vapor to air (making the air humid) reduces the density of the air, which may at first appear counter-intuitive.
This occurs because the molecular mass of water (18 g/mol) is less than the molecular mass of dry air (around 29 g/mol). For any gas, at a given temperature and pressure, the number of molecules present is constant for a particular volume (see Avogadro's Law). So when water molecules (vapor) are added to a given volume of air, the dry air molecules must decrease by the same number, to keep the pressure or temperature from increasing. Hence the mass per unit volume of the gas (its density) decreases.
The density of humid air may be calculated as a mixture of ideal gases. In this case, the partial pressure of water vapor is known as the vapor pressure. Using this method, error in the density calculation is less than 0.2% in the range of −10 °C to 50 °C. The density of humid air is found by:
where:
- Density of the humid air (kg/m³)
- Partial pressure of dry air (Pa)
- Specific gas constant for dry air, 287.058 J/(kg·K)
- Temperature (K)
- Pressure of water vapor (Pa)
- Specific gas constant for water vapor, 461.495 J/(kg·K)
- Molar mass of dry air, 0.028964 (kg/mol)
- Molar mass of water vapor, 0.018016 (kg/mol)
- Universal gas constant, 8.314 J/(K·mol)
The vapor pressure of water may be calculated from the saturation vapor pressure and relative humidity. It is found by:
Where:
The saturation vapor pressure of water at any given temperature is the vapor pressure when relative humidity is 100%. One formula [2] used to find the saturation vapor pressure is:
where T is in degrees C. Note:
- This will give a result in hPa (100 Pa, equivalent to the older unit millibar, 1 mbar = 0.001 bar = 0.1 kPa)
- is found considering partial pressure, resulting in:
Where p simply denotes the observed absolute pressure.
Altitude
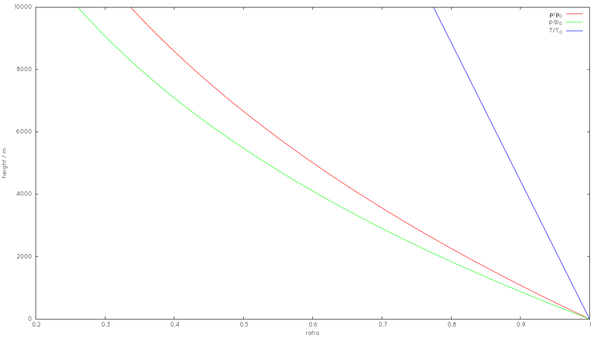
To calculate the density of air as a function of altitude, one requires additional parameters. They are listed below, along with their values according to the International Standard Atmosphere, using the universal gas constant instead of the specific one:
- sea level standard atmospheric pressure p0 = 101.325 kPa
- sea level standard temperature T0 = 288.15 K
- Earth-surface gravitational acceleration g = 9.80665 m/s2.
- temperature lapse rate L = 0.0065 K/m
- ideal (universal) gas constant R = 8.31447 J/(mol·K)
- molar mass of dry air M = 0.0289644 kg/mol
Temperature at altitude h meters above sea level is approximated by the following formula (only valid inside the troposphere):
The pressure at altitude h is given by:
Density can then be calculated according to a molar form of the ideal gas law:
where M is molar mass, R is the ideal gas constant, and T is absolute temperature. Note that p must be in Pa and not the kPa above.