Bounded mean oscillation
Hostgator is a large independently possessed hosting business operating from several state of the art centers in Dallas, Texas. Hostgator was founded in 2002, because then they have actually expanded rapidly and currently host over 400,000 websites.
Hostgator offers numerous different hosting packages, and deal with a broad array of customers. From the first time webmaster who needs simple, stress cost-free hosting for their personal website; all the way with to big corporations, who require specialist devoted hosting services.
Hostgator's hosting packages can be split into 3 teams; basic shared hosting plans (suitable for the huge bulk people), reseller hosting strategies (these are generally for people and companies that want to "resell" their account resources to consumers of their own), and lastly committed server strategies (these accounts give client their own server, so they don't have to share its resources with anyone else). Really few of us will every need a devoted server so this testimonial will focus on the shared hosting strategies that Hostgator offer.
Attributes
Hostgator's 3 major shared hosting plans are named: "Hatchling" (the entry level plan priced at $6.95 / month), "Child" (this is the most popular plan, and it is likely to satisfy the requirements of a really wide range of customers), and "Swamp" (similar as the "Baby" plan, however with increases in bandwidth and disk space, priced at $14.95 / month).
For a complete list of features and a side by side contrast of all hosting strategies you should see Hostgator's website below. Below is a testimonial of the most vital features of the "Baby" strategy, this is most likely the most ideal plan for the majority of users, and it is our favorite plan.
Disk space 100GB - This amount has actually been just recently upgraded by Hostgator from 5GB to a huge 100GB. All users are most likely to discover it impossible to tire this quantity of disk space.
Bandwidth 1000GB/month - Also enhanced is the bandwidth allotment, from 75GB to a relatively extreme 1000GB. Once again virtually absolutely no opportunity of making use of all that, but it's good to understand that you absolutely won't be dealing with additional costs for reviewing your limitation, even if you have an extremely hectic internet site(s).
Website Studio website home builder - This is an excellent free program that permits you to build your site from scratch. You have over 500 design templates and color schemes to select from. You require no HTML experience, or code writing understanding. It provides the most basic possible method of making an expert looking site in a really short area of time. Nonetheless, there is no have to take my word for it, as Hostgator offers us with a trial version of the software application on their internet site!
Unrestricted add-on domains - This truly is the stand out function of this hosting plan (the "Hatchling" strategy just permits 1 domain), and allows you to host as lots of internet sites as you like on a single account, at no additional expense. This permits you to make full use of your massive bandwidth and disk space allowances, and host numerous internet sites at a portion of the regular cost.
99.9 % Uptime guarantee - This generally tells us that Hostgator is a significant host offering a trusted service. If uptime drops below this figure in any provided month then you do not spend for that months hosting, it's as basic as that! We would never ever think about making use of a host that did not provide a strong uptime assurance; this is simply because there is only one great reason why a host will not offer an uptime assurance - undependable uptime!
30 Day money back guarantee - This has become a pretty conventional function in the webhosting community, though it's good to have for added assurance.
Immediate setup - Many hosting providers take 24-48 hours to setup your account however Hostgator guarantees to have you up and running in under 15 minutes (they do not charge a setup charge either)!
Endless MySQL databases - This is really helpful because each Fantastico (see below) script requires its own MySQL database.
Fantastico DeLuxe - This remarkable program permits you to instantly install over 50 scripts through your control board. Scripts include blog sites, forums, galleries, buying carts, and more.
If you cherished this article and also you would like to receive more info pertaining to Hostgator Coupons generously visit our own web site. Unrestricted e-mail accounts - Permits you to have as many, or as few, different email addresses as you like.
It contains numerous features and offers good performance. Best of all, there is a complete working demo on the Hostgator website, so you can test it out yourself!
Customer service and technical support
Hostgator offers us 24/7 phone support, and live online chat. The fact that you are provided 2 options to receive instantaneous technical support at any time of the day is excellent. Our experience has actually always been really good when speaking to Hostgator, their operatives are extremely respectful and most significantly they appear to understand their things when dealing with technical issues.
Performance
The efficiency from Hostgator's servers is outstanding! Hostgator location much tighter limitations on the variety of websites sharing the exact same server compared with most various other shared hosting carriers. This offers higher dependability since less stress is put on the servers; and it also greatly enhances the rate at which your web pages run.
Server efficiency is an additional one of the essential areas where Hostgator distinguish themselves from the crowd of various other host.
Our decision
Overall there is so much to such as about the method Hostgator does company, they actually do appear to have a great grasp on what the typical customer requires from a web hosting provider. Rarely do you come across reports of unhappy Hostgator customers, and after hosting with them ourselves we now understand why! At simply $9.95 / month for the "Baby" strategy (which includes limitless domains); anyone looking to host more than one internet site has a rather easy decision to make.
Template:Conjugate variables (thermodynamics)
Entropy is a property of thermodynamical systems invented by Rudolf Clausius who named it from the Greek word τρoπή, "transformation". Later Ludwig Boltzmann described the entropy as a measure of the number of possible microscopic configurations Ω of the individual atoms and molecules of the system (microstates) which comply with the macroscopic state (macrostate) of the system. Boltzmann then went on to show that klnΩ was equal to the thermodynamic entropy. The factor k has since been known as Boltzmann's constant.
Introduction

In a thermodynamic system pressure differences, density differences, and temperature differences all tend to equalize over time. For example, consider a room containing a glass of melting ice as one system. The difference in temperature between the warm room and the cold glass of ice and water is equalized as heat from the room is transferred to the cooler ice and water mixture. Over time the temperature of the glass and its contents and the temperature of the room achieve balance. The entropy of the room has decreased. However, the entropy of the glass of ice and water has increased more than the entropy of the room has decreased. In an isolated system, such as the room and ice water taken together, the dispersal of energy from warmer to cooler regions always results in a net increase in entropy. Thus, when the system of the room and ice water system has reached temperature equilibrium, the entropy change from the initial state is at its maximum. The entropy of the thermodynamic system is a measure of how far the equalization has progressed.
There are many irreversible processes that result in an increase of the entropy. See: Entropy production. One of them is mixing of two or more different substances. The mixing is accompanied by the entropy of mixing. If the substances originally are at the same temperature and pressure, there will be no net exchange of heat or work in many important cases, such as mixing of ideal gases. The entropy increase will be entirely due to the mixing of the different substances.[1]
From a macroscopic perspective, in classical thermodynamics, the entropy is a state function of a thermodynamic system: that is, a property depending only on the current state of the system, independent of how that state came to be achieved. Entropy is a key ingredient of the Second law of thermodynamics, which has important consequences e.g. for the performance of heat engines, refrigerators, and heat pumps.
Definition
According to the Clausius equality, for a closed homogeneous system, in which only reversible processes take place,
With T being the uniform temperature of the closed system and delta Q the incremental reversible transfer of heat energy into that system.
That means the line integral is path independent.
So we can define a state function S, called entropy, which satisfies
Entropy measurement
For simplicity, we examine a uniform closed system, whose thermodynamic state is determined by its temperature T and pressure P. A change in entropy can be written as
The first contribution depends on the heat capacity at constant pressure CP through
This is the result of the definition of the heat capacity by δQ = CPdT and TdS = δQ. For rewriting the second term we use one of the Maxwell relations
and the definition of the volumetric thermal-expansion coefficient
so that
With this expression the entropy S at arbitrary P and T can be related to the entropy S0 at some reference state at P0 and T0 according to
In classical thermodynamics the entropy of the reference state can be put equal to zero at any convenient temperature and pressure. E.g., for pure substances, one can take the entropy of the solid at the melting point at 1 bar equal to zero. From a more fundamental point of view, the third law of thermodynamics suggests that there is a preference to take S = 0 at T = 0 (absolute zero) for perfectly ordered materials such as crystals.
In order to determine S(P,T) we followed a specific path in the P-T diagram: first we integrated over T at constant pressure P0, so that dP=0, and in the second integral we integrated over P at constant temperature T, so that dT=0. As the entropy is a function of state the result is independent of the path.
The above relation shows that the determination of the entropy requires knowledge of the heat capacity and the equation of state (which is the relation between P,V, and T of the substance involved). Normally these are complicated functions and numerical integration is needed. In simple cases it is possible to get analytical expressions for the entropy. E.g., in the case of an ideal gas, the heat capacity is constant and the ideal-gas law PV = nRT gives that αVV = V/T = nR/p, with n the number of moles and R the molar ideal-gas constant. So, the molar entropy of an ideal gas is given by
In this expression CP now is the molar heat capacity.
The entropy of inhomogeneous systems is the sum of the entropies of the various subsystems. The laws of thermodynamics hold rigorously for inhomogeneous systems even though they may be far from internal equilibrium. The only condition is that the thermodynamic parameters of the composing subsystems are (reasonably) well-defined.

Temperature-entropy diagrams
Nowadays the entropy values of important substances can be obtained via commercial software in tabular form or as diagrams. One of the most common diagrams is the temperature-entropy diagram (Ts-diagram). An example is Fig.2 which is the Ts-diagram of nitrogen.[2] It gives the melting curve and saturated liquid and vapor values together with isobars and isenthalps.
Entropy change in irreversible transformations
DTZ's public sale group in Singapore auctions all forms of residential, workplace and retail properties, outlets, homes, lodges, boarding homes, industrial buildings and development websites. Auctions are at present held as soon as a month.
We will not only get you a property at a rock-backside price but also in an space that you've got longed for. You simply must chill out back after giving us the accountability. We will assure you 100% satisfaction. Since we now have been working in the Singapore actual property market for a very long time, we know the place you may get the best property at the right price. You will also be extremely benefited by choosing us, as we may even let you know about the precise time to invest in the Singapore actual property market.
The Hexacube is offering new ec launch singapore business property for sale Singapore investors want to contemplate. Residents of the realm will likely appreciate that they'll customize the business area that they wish to purchase as properly. This venture represents one of the crucial expansive buildings offered in Singapore up to now. Many investors will possible want to try how they will customise the property that they do determine to buy by means of here. This location has offered folks the prospect that they should understand extra about how this course of can work as well.
Singapore has been beckoning to traders ever since the value of properties in Singapore started sky rocketing just a few years again. Many businesses have their places of work in Singapore and prefer to own their own workplace area within the country once they decide to have a everlasting office. Rentals in Singapore in the corporate sector can make sense for some time until a business has discovered a agency footing. Finding Commercial Property Singapore takes a variety of time and effort but might be very rewarding in the long term.
is changing into a rising pattern among Singaporeans as the standard of living is increasing over time and more Singaporeans have abundance of capital to invest on properties. Investing in the personal properties in Singapore I would like to applaud you for arising with such a book which covers the secrets and techniques and tips of among the profitable Singapore property buyers. I believe many novice investors will profit quite a bit from studying and making use of some of the tips shared by the gurus." – Woo Chee Hoe Special bonus for consumers of Secrets of Singapore Property Gurus Actually, I can't consider one other resource on the market that teaches you all the points above about Singapore property at such a low value. Can you? Condominium For Sale (D09) – Yong An Park For Lease
In 12 months 2013, c ommercial retails, shoebox residences and mass market properties continued to be the celebrities of the property market. Models are snapped up in report time and at document breaking prices. Builders are having fun with overwhelming demand and patrons need more. We feel that these segments of the property market are booming is a repercussion of the property cooling measures no.6 and no. 7. With additional buyer's stamp responsibility imposed on residential properties, buyers change their focus to commercial and industrial properties. I imagine every property purchasers need their property funding to understand in value.
DTZ's public sale group in Singapore auctions all forms of residential, workplace and retail properties, outlets, homes, lodges, boarding homes, industrial buildings and development websites. Auctions are at present held as soon as a month.
We will not only get you a property at a rock-backside price but also in an space that you've got longed for. You simply must chill out back after giving us the accountability. We will assure you 100% satisfaction. Since we now have been working in the Singapore actual property market for a very long time, we know the place you may get the best property at the right price. You will also be extremely benefited by choosing us, as we may even let you know about the precise time to invest in the Singapore actual property market.
The Hexacube is offering new ec launch singapore business property for sale Singapore investors want to contemplate. Residents of the realm will likely appreciate that they'll customize the business area that they wish to purchase as properly. This venture represents one of the crucial expansive buildings offered in Singapore up to now. Many investors will possible want to try how they will customise the property that they do determine to buy by means of here. This location has offered folks the prospect that they should understand extra about how this course of can work as well.
Singapore has been beckoning to traders ever since the value of properties in Singapore started sky rocketing just a few years again. Many businesses have their places of work in Singapore and prefer to own their own workplace area within the country once they decide to have a everlasting office. Rentals in Singapore in the corporate sector can make sense for some time until a business has discovered a agency footing. Finding Commercial Property Singapore takes a variety of time and effort but might be very rewarding in the long term.
is changing into a rising pattern among Singaporeans as the standard of living is increasing over time and more Singaporeans have abundance of capital to invest on properties. Investing in the personal properties in Singapore I would like to applaud you for arising with such a book which covers the secrets and techniques and tips of among the profitable Singapore property buyers. I believe many novice investors will profit quite a bit from studying and making use of some of the tips shared by the gurus." – Woo Chee Hoe Special bonus for consumers of Secrets of Singapore Property Gurus Actually, I can't consider one other resource on the market that teaches you all the points above about Singapore property at such a low value. Can you? Condominium For Sale (D09) – Yong An Park For Lease
In 12 months 2013, c ommercial retails, shoebox residences and mass market properties continued to be the celebrities of the property market. Models are snapped up in report time and at document breaking prices. Builders are having fun with overwhelming demand and patrons need more. We feel that these segments of the property market are booming is a repercussion of the property cooling measures no.6 and no. 7. With additional buyer's stamp responsibility imposed on residential properties, buyers change their focus to commercial and industrial properties. I imagine every property purchasers need their property funding to understand in value.
We now consider inhomogeneous systems in which internal transformations (processes) can take place. If we calculate the entropy S1 before and S2 after such an internal process the Second Law of Thermodynamics demands that S2 ≥ S1 where the equality sign holds if the process is reversible. The difference Si = S2 - S1 is the entropy production due to the irreversible process. The Second law demands that the entropy of an isolated system cannot decrease.
Suppose a system is thermally and mechanically isolated from the environment (isolated system). For example, consider an insulating rigid box divided by a movable partition into two volumes, each filled with gas. If the pressure of one gas is higher, it will expand by moving the partition, thus performing work on the other gas. Also, if the gases are at different temperatures, heat can flow from one gas to the other provided the partition allows heat conduction. Our above result indicates that the entropy of the system as a whole will increase during these processes. There exists a maximum amount of entropy the system may possess under the circumstances. This entropy corresponds to a state of stable equilibrium, since a transformation to any other equilibrium state would cause the entropy to decrease, which is forbidden. Once the system reaches this maximum-entropy state, no part of the system can perform work on any other part. It is in this sense that entropy is a measure of the energy in a system that cannot be used to do work.
An irreversible process degrades the performance of a thermodynamic system, designed to do work or produce cooling, and results in entropy production. The entropy generation during a reversible process is zero. Thus entropy production is a measure of the irreversibility and may be used to compare engineering processes and machines.
Thermal machines
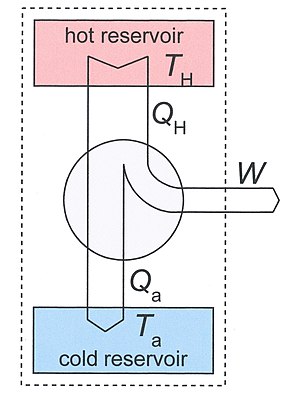
Clausius' identification of S as a significant quantity was motivated by the study of reversible and irreversible thermodynamic transformations. A heat engine is a thermodynamic system that can undergo a sequence of transformations which ultimately return it to its original state. Such a sequence is called a cyclic process, or simply a cycle. During some transformations, the engine may exchange energy with its environment. The net result of a cycle is
- mechanical work done by the system (which can be positive or negative, the latter meaning that work is done on the engine),
- heat transferred from one part of the environment to another. In the steady state, by the conservation of energy, the net energy lost by the environment is equal to the work done by the engine.
If every transformation in the cycle is reversible, the cycle is reversible, and it can be run in reverse, so that the heat transfers occur in the opposite directions and the amount of work done switches sign.
Heat engines
Consider a heat engine working between two temperatures TH and Ta. With Ta we have ambient temperature in mind, but, in principle it may also be some other low temperature. The heat engine is in thermal contact with two heat reservoirs which are supposed to have a very large heat capacity so that their temperatures do not change significantly if heat QH is removed from the hot reservoir and Qa is added to the lower reservoir. Under normal operation TH > Ta and QH, Qa, and W are all positive.
As our thermodynamical system we take a big system which includes the engine and the two reservoirs. It is indicated in Fig.3 by the dotted rectangle. It is inhomogeneous, closed (no exchange of matter with its surroundings), and adiabatic (no exchange of heat with its surroundings). It is not isolated since per cycle a certain amount of work W is produced by the system given by the First law of thermodynamics
We used the fact that the engine itself is periodic, so its internal energy has not changed after one cycle. The same is true for its entropy, so the entropy increase S2 - S1 of our system after one cycle is given by the reduction of entropy of the hot source and the increase of the cold sink. The entropy increase of the total system S2 - S1 is equal to the entropy production Si due to irreversible processes in the engine so
The Second law demands that Si ≥ 0. Eliminating Qa from the two relations gives
The first term is the maximum possible work for a heat engine, given by a reversible engine, as one operating along a Carnot cycle. Finally
This equation tells us that the production of work is reduced by the generation of entropy. The term TaSi gives the lost work, or dissipated energy, by the machine.
Correspondingly, the amount of heat, discarded to the cold sink, is increased by the entropy generation
These important relations can also be obtained without the inclusion of the heat reservoirs. See the Article on entropy production.
Refrigerators
The same principle can be applied to a refrigerator working between a low temperature TL and ambient temperature. The schematic drawing is exactly the same as Fig.3 with TH replaced by TL, QH by QL, and the sign of W reversed. In this case the entropy production is
and the work, needed to extract heat QL from the cold source, is
The first term is the minimum required work, which corresponds to a reversible refrigerator, so we have
i.e., the refrigerator compressor has to perform extra work to compensate for the dissipated energy due to irreversible processes which lead to entropy production.
See also
- Entropy
- Enthalpy
- Entropy production
- Fundamental thermodynamic relation
- Thermodynamic free energy
- History of entropy
- Entropy (statistical views)
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
Further reading
- E.A. Guggenheim Thermodynamics, an advanced treatment for chemists and physicists North-Holland Publishing Company, Amsterdam, 1959.
- C. Kittel and H. Kroemer Thermal Physics W.H. Freeman and Company, New York, 1980.
- Goldstein, Martin, and Inge F., 1993. The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction at a lower level than this entry.
- ↑ See, e.g., Notes for a “Conversation About Entropy” for a brief discussion of both thermodynamic and "configurational" ("positional") entropy in chemistry.
- ↑ Figure composed with data obtained with RefProp, NIST Standard Reference Database 23

















