Bimetallic strip: Difference between revisions
en>Wtshymanski restore moving pictures Undid revision 504287256 by 117.219.25.150 (talk) |
en>HMSSolent m Reverted 2 edits by 195.195.244.165 identified as test/vandalism using STiki |
||
| Line 1: | Line 1: | ||
{{see also|Molecular orbital}} | |||
In [[chemistry]], '''molecular orbital (MO) theory''' is a method for determining molecular structure in which [[electron]]s are not assigned to individual [[chemical bond|bond]]s between [[atom]]s, but are treated as moving under the influence of the [[Atomic nucleus|nuclei]] in the whole molecule.<ref>{{cite book|author=Daintith, J. |title=Oxford Dictionary of Chemistry|location=New York | publisher=Oxford University Press|year=2004|isbn=0-19-860918-3}}</ref> Because electrons are the fundamental constituents of matter involved in bonding, their involvement in bonding has been studied constantly by chemists. Electrons are shared among individual atoms in a molecule to form covalent chemical bonds. Generally, up to three bonds can form between atoms in a molecule. Single, or sigma covalent bonds result from the interaction between the nuclei of two discrete atoms; multiple bonds then can result due to the additional formation of pi bonds between overlapping orbitals of like symmetries.<ref>“Sigma and Pi Bonds.” 11/8/13. <http://www.kentchemistry.com/links/bonding/sigmapi.htm></ref> Electrons in sigma bonds are located between the nuclei, while electrons in pi bonds are delocalized in regions above and below the nuclei.<ref>“Sigma and Pi Bonds.” 11/8/13. <http://www.kentchemistry.com/links/bonding/sigmapi.htm></ref> | |||
The spatial and energetic properties of electrons within atoms are fixed by quantum mechanics to form orbitals that contain these electrons.<ref name="ICL">[http://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html Introduction to Molecular Orbital Theory] - Imperial College London</ref> While atomic orbitals contain electrons within a single atom, molecular orbitals, which surround a number of atoms in a molecule, contain [[valence electrons]] between atoms.<ref name="ICL" /> Molecular orbital theory, which was proposed in the early twentieth century, revolutionized the study of bonding by approximating the positions of bonded electrons—the molecular orbitals—as Linear Combinations of Atomic Orbitals ([[LCAO]]).<ref>“Linear combination of atomic orbitals.” 11/8/13. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_14/node1.html>.</ref> These approximations are made by applying the Density Functional Theory (DFT) and Hartree-Fock (HF) models to the Schrödinger equation.<ref name="TU-Darmstadt">“Molecular Orbitals of Benzene.” 11/8/13. < http://csi.chemie.tu-darmstadt.de/ak/immel/script/redirect.cgi?filename=http://csi.chemie.tu-darmstadt.de/ak/immel/tutorials/orbitals/molecular/benzene.html>.</ref> | |||
MO theory applies the wavelike behavior of electrons as predicted by quantum mechanics, in that electrons no longer deterministically are given defined coordinates, but rather are given probable locations according to the mathematical wavefunctions defining all the possible positions of the electrons. These wavefunctions, or electron [[eigenstates]], quantitatively describe the atomic orbital basis in which an electron temporarily can reside. Molecular orbitals result from the mixing of these atomic orbitals. In this theory, each molecule has a set of [[molecular orbital]]s, in which it is assumed that the molecular orbital [[wave function]] ''ψ<sub>j</sub>'' can be written as a simple weighted sum of the n constituent [[atomic orbital]]s ''χ<sub>i</sub>'', according to the following equation:<ref>{{cite book|author=Licker, Mark, J. |title=McGraw-Hill Concise Encyclopedia of Chemistry|location=New York | publisher=McGraw-Hill|year=2004|isbn=0-07-143953-6}}</ref> | |||
:<math> \psi_j = \sum_{i=1}^{n} c_{ij} \chi_i.</math> | |||
==Connection to MO diagrams and molecular geometry== | |||
Using molecular orbital theory, a [[molecular orbital diagram]] can be constructed that features the individual atomic basis orbitals and the molecular orbitals resulting from atomic interactions to form molecules. All atomic s, p, d and f orbitals are assigned relative electronic energies, and orbital interactions are predicted by the relative proximity in the energies of the individual atomic orbitals. The closer the atomic orbitals are in energy, the stronger and more likely their molecular orbital interaction is. Through qualitative molecular orbital analysis through a molecular orbital diagram, the chemist is able to display the resulting molecular orbitals stemming from atomic interaction based on their relative energies and assign bonding, nonbonding and/or antibonding character to each molecular orbital.<ref name="Tarr 2013">Miessler and Tarr (2013), Inorganic Chemistry, 5th ed, 117-165, 475-534.</ref> After these assignments are made, the chemist is able to identify the Highest Occupied Molecular Orbital (HOMO) that contains electrons and the Lowest Unoccupied Molecular Orbital (LUMO) that does not contain electrons. The difference in energy between these two [[frontier orbital]]s can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution.<ref name="Griffth 1957">Griffith, J.S. and L.E. Orgel. Ligand Field Theory. Q. Rev. Chem. Soc. 1957, 11, 381-383. <http://pubs.rsc.org/en/content/articlelanding/1957/qr/qr9571100381#!divAbstract>.</ref> | |||
The known geometries of all molecules and their (s,p,d,f) orbitals, as predicted by [[VSEPR theory]], are used in conjunction with molecular orbital theory in determining whether select orbitals of an atomic basis are used for bonding, nonbonding, or antibonding interactions within a molecule. Bonding orbitals participate in strengthening the interaction between two atoms of a molecule; nonbonding orbitals exclusively are associated with one atom and usually represent a lone pair; antibonding orbitals participate in weakening the interaction between two atoms of a molecule.<ref name="UCDavis ChemWiki" >“How to Build Molecular Orbitals.” UC Davis ChemWiki. 11/8/13. <http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/Molecular_Orbital_Theory/How_to_Build_Molecular_Orbitals>.</ref> Defining this interaction through [[Molecular orbital|symmetry adapted linear combinations]] (SALC’s), in which the geometry of valence orbital interaction is illustrated, allows the chemist to justify the assignment of a particular molecular orbital as either bonding, nonbonding or antibonding. SALC’s of a molecule can be drawn by knowing either the VSEPR geometry or point group symmetry of that molecule.<ref name="Tarr 2013"/> | |||
==Quantitative applications== | |||
One may determine c<sub>ij</sub> coefficients numerically by substituting this equation into the [[Schrödinger equation]] and applying the [[variational principle]]. The variational principle is a mathematical technique used in quantum mechanics to build up the coefficients of each atomic orbital basis. A larger coefficient means that the orbital basis is composed more of that particular contributing atomic orbital—hence, the molecular orbital is best characterized by that type.<ref>“Linear combination of atomic orbitals.” 11/8/13. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_14/node1.html>.</ref> This method of quantifying orbital contribution as Linear Combinations of Atomic Orbitals is used in [[computational chemistry]]. An additional [[unitary transformation]] can be applied on the system to accelerate the convergence in some computational schemes. Molecular orbital theory was seen as a competitor to [[valence bond theory]] in the 1930s, before it was realized that the two methods are closely related and that when extended they become equivalent. Valence bond theory further describes the composition of each orbital in a molecule. Unlike MO theory, valence bond theory postulates that molecular orbitals are hybridized and contain a certain percentage of s, p and d character as determined by the number of bonds to the central atom (i.e. tetrahedral complexes feature four sp3 hybridized orbitals that contain 25% s character).<ref name="CSB SJU" /> It similarly proposes that the strength of bonds increases with increasing overlap between atomic orbitals.<ref name="CSB SJU" /> | |||
==History== | |||
{{main|History of quantum mechanics}} | |||
Molecular orbital theory was developed, in the years after valence bond theory had been established (1927), primarily through the efforts of [[Friedrich Hund]], [[Robert Mulliken]], [[John C. Slater]], and [[John Lennard-Jones]].<ref>{{cite book | last = Coulson | first = Charles, A. | title = Valence | publisher = Oxford at the Clarendon Press | year = 1952}}</ref> MO theory was originally called the Hund-Mulliken theory.<ref name="Mulliken" >{{cite press release |url=http://nobelprize.org/nobel_prizes/chemistry/laureates/1966/mulliken-lecture.pdf |origyear=1966 |year=1972 |format=pdf |title=Spectroscopy, Molecular Orbitals, and Chemical Bonding |series=Nobel Lectures, Chemistry 1963-1970 |publisher=Elsevier Publishing Company |location=Amsterdam}}</ref> The word ''orbital'' was introduced by Mulliken in 1932.<ref name="Mulliken" /> By 1933, the molecular orbital theory had been accepted as a valid and useful theory.<ref>{{cite journal |url=http://www.quantum-chemistry-history.com/LeJo_Dat/LJ-Hall1.htm Lennard-Jones Paper of 1929 |title=Foundations of Molecular Orbital Theory. |first=George G |last=Hall |journal=Advances in Quantum Chemistry |volume=22 |issn=0065-3276 |ISBN=978-0-12-034822-0 |doi=10.1016/S0065-3276(08)60361-5|bibcode = 1991AdQC...22....1H }}</ref> According to German physicist and physical chemist [[Erich Hückel]], the first quantitative use of molecular orbital theory was the 1929 paper of Lennard-Jones.<ref>{{cite journal |last=Hückel |first=Erich |year=1934 |journal=Trans. Faraday Soc. |volume=30 |doi=10.1039/TF9343000040 |title=Theory of free radicals of organic chemistry |pages=40–52}}</ref> The first accurate calculation of a molecular orbital wavefunction was that made by [[Charles Coulson]] in 1938 on the hydrogen molecule.<ref>{{citation | |||
|last=Coulson |first=C.A. |author-link=Charles_Coulson |title=Self-consistent field for molecular hydrogen |journal=Mathematical Proceedings of the Cambridge Philosophical Society |volume=34 |issue=2 |pages=204–212 |year=1938 |doi=10.1017/S0305004100020089|bibcode = 1938PCPS...34..204C }}</ref> By 1950, [[molecular orbital]]s were completely defined as [[eigenfunctions]] (wave functions) of the self-consistent field [[Hamiltonian (quantum mechanics)|Hamiltonian]] and it was at this point that molecular orbital theory became fully rigorous and consistent.<ref>{{cite journal |doi=10.1098/rspa.1950.0104 |last=Hall |first=G.G. |journal=Proc. Roy. Soc. A |volume=202 |pages=336–344 |issue=1070 |date=7 August 1950 |title= The Molecular Orbital Theory of Chemical Valency. VI. Properties of Equivalent Orbitals|url=http://rspa.royalsocietypublishing.org/content/202/1070/336.full.pdf+html |format=pdf|bibcode = 1950RSPSA.202..336H }}</ref> This rigorous approach is known as the [[Hartree–Fock method]] for molecules although it had its origins in calculations on atoms. In calculations on molecules, the molecular orbitals are expanded in terms of an [[basis set (chemistry)|atomic orbital basis set]], leading to the [[Roothaan equations]].<ref name="Frank">{{cite book |first=Frank |last=Jensen |title=Introduction to Computational Chemistry |publisher=John Wiley and Sons |year=1999 |isbn=978-0-471-98425-2}}</ref> This led to the development of many [[ab initio quantum chemistry methods]]. In parallel, molecular orbital theory was applied in a more approximate manner using some empirically derived parameters in methods now known as [[semi-empirical quantum chemistry methods]].<ref name="Frank"/> | |||
==Application to ligand field theory== | |||
{{main|Ligand field theory}} | |||
Ligand field theory resulted from combining the principles laid out in molecular orbital theory and [[crystal field theory]], which describes the loss of degeneracy of frontier orbitals in transition metal complexes. Griffith and Orgel<ref name="Griffth 1957" /> championed ligand field theory as a more accurate description of transition metal coordinated compounds. They used the electrostatic principles established in crystal field theory to describe transition metal ions in solutions, and they used molecular orbital theory to explain the differences in transition metal complex interactions. In their paper, they propose that the chief consequence of differences in colors produced by transition metal complexes in solution is incomplete d orbital subshells.<ref name="Griffth 1957" /> That is, the d orbitals of transition metals unoccupied by electrons participate in the bonding that influences the colors they emit in solution. Through ligand field theory, the connection between d orbital energy of transition metal complexes and the character of ligands bound to the transition metal was established. Based on the geometric symmetry between the various d orbitals (d<sub>z<sup>2</sup></sub>, d<sub>x<sup>2</sup>-y<sup>2</sup></sub>, d<sub>xz</sub>, d<sub>yz</sub>, d<sub>xy</sub>) of the metal and its associated ligands, the relative energies of the d orbitals could be qualitatively and quantitatively assessed. Ligand field theory, therefore, explains how transition metal complexes adjust structurally to reduce degeneracy among d orbital energies. This phenomenon is known as the [[Jahn-Teller effect]]. Because d orbital energies are affected differently when surrounded by a field of neighboring ligands, as determined by SALC’s, they separately are raised or lowered in energy based on the intensity that their geometries permit them to interact with the ligands.<ref name="Griffth 1957" /> Thus, ligand field theory allows the chemist to determine d orbital energy splittings in transition metal complexes based on the VSEPR-assigned geometries of these compounds. For instance, octahedral complexes are known from SALC’s to feature the greatest interaction between the d<sub>z<sup>2</sup></sub> frontier orbital and the ligands. Relative interaction intensities between the d orbitals and ligands for other geometries, such as square planar and tetrahedral, also are known.<ref name="Griffth 1957" /> | |||
[[File:D orbitals.gif|thumbnail|Figure 1. A geometric description of the d orbitals.]] | |||
The potential of the d orbitals to interact with the ligands affects the relative energies between them.<ref name="Griffth 1957" /> Due to the toroidal shape of the d<sub>z<sup>2</sup></sub> orbital, it is capable of interacting with ligands in both the axial and equatorial orientations, resulting in its energy being comparatively raised relative to the other d orbitals due to the repulsive interaction with the ligands. Furthermore, in tetrahedral and octahedral complexes, the stronger overall interaction of the d<sub>z<sup>2</sup></sub> and d<sub>x<sup>2</sup>-y<sup>2</sup></sub> orbitals with the ligands induces the separation of these two d orbitals from the d<sub>xz</sub>, d<sub>yz</sub>, and d<sub>xy</sub> orbtials. This results in d orbital splitting into a doubly degenerate (e<sub>g</sub>) state and a triply degenerate (t<sub>2g</sub>) state.<ref name="Griffth 1957" /> The energy splitting of the two d orbital states is a predictor of electron spin state, stability, and luminescent color emitted by the complex in solution. Electrostatic theory can be used to predict electronic transitions between the ground-state t<sub>2g</sub> and excited state e<sub>g</sub> energy levels.<ref name="Griffth 1957" /> Complexes in which electrons fill the t<sub>2g</sub> orbitals prior to filling the e<sub>g</sub> orbitals are referred to as low-spin complexes; conversely, complexes in which electrons will fill the e<sub>g</sub> orbitals prior to completely filling the t<sub>2g</sub> orbitals, thereby maximizing the number of electron parallel spins, are referred to as high-spin complexes.<ref name="Tarr 2013"/> | |||
==Overview== | |||
[[Molecular orbital]] (MO) theory uses a [[linear combination of atomic orbitals]] (LCAO) to represent molecular orbitals resulting from bonds between atoms.<ref name="ICL" /> These are often divided into bonding orbitals, [[antibonding|anti-bonding]] orbitals, and [[non-bonding orbital]]s. The Schrödinger equation—an application of the wave equation to quantum mechanics used to build up the orbital bases of an atom or molecule—can be solved in three-dimensions to determine the shape of each molecular orbital.<ref name="ICL" /> A [[molecular orbital]] is a Schrödinger orbital that includes two or more nuclei. If this orbital is of the type in which the electron(s) in the orbital have a higher probability of being ''between'' nuclei than elsewhere, the orbital will be a bonding orbital, and will tend to hold the nuclei together.<ref name="Tarr 2013"/> A bonding orbital is of lower energy than the two contributing atomic bases, and the percentage composition of each atomic orbital to the molecular orbital is determined by molecular orbital theory. For bonding orbitals, the atomic orbital of lower energy contributes more to the molecular orbital, causing the molecular orbital to more closely resemble it.<ref>A bonding orbital is of lower energy than the two contributing atomic bases, and the percentage composition of each atomic orbital to the molecular orbital is determined by molecular orbital theory. For bonding orbitals, the atomic orbital of lower energy contributes more to the molecular orbital, causing the molecular orbital to more closely resemble it.</ref> If the electrons tend to be present in a molecular orbital in which they spend more time elsewhere than between the nuclei, the orbital will function as an [[antibonding orbital|anti-bonding orbital]] and will actually weaken the bond. Conversely, an antibonding orbital will more closely resemble the higher energy atomic basis.<ref name="Tarr 2013"/> Electrons in non-bonding orbitals tend to be in deep orbitals (nearly [[atomic orbital]]s) associated almost entirely with one nucleus or the other, and thus they spend equal time within as they do between nuclei. These electrons neither contribute to nor detract from bond strength and thus are best described as lone pairs.<ref name="Tarr 2013"/> | |||
Molecular orbitals are further divided according to the types of atomic orbitals combining to form a bond. These orbitals are results of electron-[[Atomic nucleus|nucleus]] interactions that are caused by the [[fundamental interaction|fundamental]] force of [[electromagnetism]]—the attraction between the positively charged nucleus and negatively charged electron.<ref>“The Four Fundamental Forces.” Oracle ThinkQuest. 11/8/13. <http://library.thinkquest.org/27930/forces.htm>.</ref> | |||
Quantum mechanics stipulates that an electron’s position is confined solely to the atomic orbital basis, characterized by s, p, d and f subshells with unique shielding parameters. Chemical substances will form a bond if their orbitals become lower in energy when they interact with each other. This decrease in energy may stem from pi backbonding of a ligand to a metal, which can stabilize a metal’s electron pair through delocalization.<ref name="Tarr 2013"/> Thus, bonding molecular orbitals have greater electron density than their component atomic orbitals. Different chemical bonds are distinguished that differ by [[electron configuration]] (electron cloud shape) and by [[energy level]]s. All atomic orbitals are characterized by a principal atomic number n, or energy level, an azimuthal quantum number l distinguishing s, p, d and f subshells, and a magnetic quantum number m defined as the range –l<m<l. In addition, all electrons are placed into orbitals and are assigned appropriate spin according to the Aufbau principle, Hund’s rule and the Pauli exclusion principle.<ref name="Tarr 2013"/> | |||
Molecular orbital diagrams are how chemists analyze the electron configuration of a molecule. They are constructed as qualitative descriptions of bonding within molecules by invoking Molecular Orbital Theory. While VSEPR and valence bond theory are applied to predict the structures of molecules,<ref name="UCDavis ChemWiki" /> MO theory is used to determine orbital interaction and valence electron configuration within the molecular orbitals of a molecule. MO diagrams incorporate the wavelike behavior of electrons as postulated by MO theory.<ref name="UCDavis ChemWiki" /> They are used to predict the shape of a molecule, as well as the energy, length and angle of each bond.<ref name="UCDavis ChemWiki" /> Upon constructing an MO diagram, the following stipulations must be met:<ref name="UCDavis ChemWiki" /> | |||
* Orbitals are built up along the y-axis from lowest to highest energy. | |||
* Atomic orbitals of the individual atoms are drawn to the left and right of the molecular orbitals, and are traced to each MO they contribute to. | |||
* Overlapping atomic orbitals contribute to molecular orbitals that possess σ, π, and/or nonbonding character. | |||
* Valence electrons from the atomic orbitals are assigned to molecular orbitals by the Pauli Exclusion Principle. | |||
A complete MO diagram therefore reveals the transformation of all the atomic orbitals into their resulting molecular orbital descriptions. MO diagrams are filled by first determining the number of valence electrons contributed by all the atoms of a molecule, and then evaluating the strength of the orbital interactions by the degree of overlap. Because σ bonds feature greater overlap than π bonds, which are usually long-range, σ and σ* bonding and antibonding orbitals, respectively, feature greater energy splitting (separation) than π and π* orbitals.<ref name="UCDavis ChemWiki" /> | |||
MO theory provides a global, delocalized perspective on chemical bonding. In MO theory, ''any'' electron in a molecule may be found ''anywhere'' in the molecule, since quantum conditions allow electrons to travel under the influence of an arbitrarily large number of nuclei, as long as they are in eigenstates permitted by certain quantum rules. Thus, when excited with the requisite amount of energy through high-frequency light or other means, electrons can transition to higher-energy—even antibonding—molecular orbitals.<ref name="ICL" /> For instance, in the simple case of a hydrogen diatomic molecule, promotion of a single electron from a bonding orbital to an antibonding orbital can occur under UV radiation. This promotion weakens the bond between the two hydrogen atoms and can lead to photodissociation—the breaking of a chemical bond due to the absorption of light.<ref>“Photodissociation Dynamics.” Kable Group. University of Sydney. 11/8/13. <http://sydney.edu.au/science/chemistry/~kable_s/projects/dynamics/>.</ref> | |||
Although in MO theory ''some'' molecular orbitals may hold electrons that are more localized between specific pairs of molecular atoms, ''other'' orbitals may hold electrons that are spread more uniformly over the molecule. Thus, overall, bonding is far more delocalized in MO theory, which makes it more applicable to resonant molecules that have equivalent non-integer bond orders than valence bond (VB) theory.<ref>“Molecular Structure and Bonding: Bonding Theories.” 11/8/13. <http://www.chem.latech.edu/~upali/chem281/notes/Ch2-MO-Theory.pdf>.</ref> For instance, MO theory is used to explain the resonant intermediate bond character of NO3-, in which the N-O bonds have a mix of single and double bond character. This makes MO theory more useful for the description of extended systems. | |||
An example is the MO description of [[benzene]], {{chem|C|6|H|6}}, which is an aromatic hexagonal ring of six carbon atoms and three double bonds. In this molecule, 24 of the 30 total valence bonding electrons—24 coming from carbon atoms and 6 coming from hydrogen atoms—are located in 12 σ (sigma) bonding orbitals, which are located mostly between pairs of atoms (C-C or C-H), similarly to the electrons in the valence bond description.<ref name="TU-Darmstadt" /> However, in benzene the remaining six bonding electrons are located in three π (pi) molecular bonding orbitals that are delocalized around the ring.<ref name="TU-Darmstadt" /> Two of these electrons are in an MO that has equal resonant contributions from all six atoms. The other four electrons are in orbitals with vertical nodes at right angles to each other. As in the VB theory, all of these six delocalized π electrons reside in a larger space that exists above and below the ring plane. All carbon-carbon bonds in benzene are chemically equivalent. In MO theory this is a direct consequence of the fact that the three molecular π orbitals combine and evenly spread the extra six electrons over six carbon atoms.<ref name="ICL" /> | |||
[[File:Benzene structure.png|thumbnail|Structure of benzene.]] | |||
In molecules such as [[methane]], {{chem|C|H|4}}, the eight valence electrons are found in four MOs that are spread out over all five atoms. However, it is possible to approximate the MOs with four localized orbitals similar in shape to the sp<sup>3</sup> hybrid orbitals predicted by VB theory.<ref name="CSB SJU" /> Linus Pauling, in 1931, hybridized the carbon 2s and 2p orbitals so that they pointed directly at the hydrogen 1s basis functions and featured maximal overlap.<ref name="CSB SJU" /> This is often adequate for [[sigma bond|σ bonds]], but is not possible for the [[pi bond|π orbitals]]. However, the delocalized MO description is more appropriate for [[ionization]] and [[spectroscopic]] predictions.<ref name="CSB SJU" /> When methane is ionized, a single electron is taken from the MO, which surrounds the whole molecule, weakening all four bonds equally. VB theory would predict that one electron is removed from an sp<sup>3</sup> orbital, resulting in the need for [[resonance (chemistry)|resonance]] between four valence bond structures, each of which has a single one-electron bond and three two-electron bonds. Thus, while the VB model predicts only one ionization energy for methane, there are two ionization energies from the lowest molecular orbitals as predicted by MO theory.<ref name="CSB SJU">“A Molecular Orbital Approach to Bonding in Methane.” 11/8/13. <http://www.users.csbsju.edu/~frioux/h2bond/MethaneMOBonding.pdf></ref> | |||
As in benzene, in substances such as [[beta carotene]], [[chlorophyll]], or [[heme]], some electrons in the π orbitals are spread out in molecular orbitals over long distances in a molecule, resulting in light absorption in lower energies (the [[visible spectrum]]), which accounts for the characteristic colours of these substances.<ref name="Griffth 1957" /> This and other spectroscopic data for molecules are better explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching.<ref name="Tarr 2013"/> The same MO principles also more naturally explain some electrical phenomena, such as high [[electrical conductivity]] in the planar direction of the hexagonal atomic sheets that exist in [[graphite]]. This results from continuous band overlap of half-filled p orbitals.<ref>“Benzene and Graphite.” 11/8/13. <http://www.seas.upenn.edu/~chem101/sschem/conduction.html#graphite>.</ref> In MO theory, "resonance" is a natural consequence of symmetry. For example, in [[graphite]], as in benzene, it is not necessary to invoke the sp<sup>2</sup> hybridization and resonance of VB theory, in order to explain electrical conduction. Instead, MO theory recognizes that some electrons in the graphite atomic sheets are completely [[delocalized electron|delocalized]] over arbitrary distances, and reside in very large molecular orbitals that cover an entire graphite sheet, and some electrons are thus as free to move and therefore conduct electricity in the sheet plane, as if they resided in a [[metal]].<ref>“Benzene and Graphite.” 11/8/13. <http://www.seas.upenn.edu/~chem101/sschem/conduction.html#graphite>.</ref> | |||
== See also == | |||
{{col-begin}} | |||
{{col-break}} | |||
*[[Ab initio quantum chemistry methods]] | |||
*[[Atomic orbital]] | |||
*[[Configuration interaction]] | |||
*[[Coupled cluster]] | |||
*[[Hartree–Fock method]] | |||
{{col-break}} | |||
*[[Molecular orbital]] | |||
*[[Molecular orbital diagram]] | |||
*[[Møller–Plesset perturbation theory]] | |||
*[[Quantum chemistry computer programs]] | |||
*[[Semi-empirical quantum chemistry methods]] | |||
*[[cis effect]] | |||
*[[Addition to pi ligands]] | |||
{{col-end}} | |||
==References== | |||
{{Reflist}} | |||
==External links== | |||
*[http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/mo.html Molecular Orbital Theory] - Purdue University | |||
*[http://www.sparknotes.com/chemistry/bonding/molecularorbital/section1.html Molecular Orbital Theory] - Sparknotes | |||
*[http://www.mpcfaculty.net/mark_bishop/molecular_orbital_theory.htm Molecular Orbital Theory] - Mark Bishop's Chemistry Site | |||
*[http://www.chem.qmul.ac.uk/software/download/mo/ Introduction to MO Theory] - Queen Mary, London University | |||
*[http://www.chm.davidson.edu/ChemistryApplets/MolecularOrbitals/index.html Molecular Orbital Theory] - a related terms table | |||
[[Category:Chemistry theories]] | |||
[[Category:Quantum chemistry]] | |||
[[Category:Chemical bonding]] | |||
Revision as of 17:59, 20 January 2014
DTZ's public sale group in Singapore auctions all forms of residential, workplace and retail properties, outlets, homes, lodges, boarding homes, industrial buildings and development websites. Auctions are at present held as soon as a month.
We will not only get you a property at a rock-backside price but also in an space that you've got longed for. You simply must chill out back after giving us the accountability. We will assure you 100% satisfaction. Since we now have been working in the Singapore actual property market for a very long time, we know the place you may get the best property at the right price. You will also be extremely benefited by choosing us, as we may even let you know about the precise time to invest in the Singapore actual property market.
The Hexacube is offering new ec launch singapore business property for sale Singapore investors want to contemplate. Residents of the realm will likely appreciate that they'll customize the business area that they wish to purchase as properly. This venture represents one of the crucial expansive buildings offered in Singapore up to now. Many investors will possible want to try how they will customise the property that they do determine to buy by means of here. This location has offered folks the prospect that they should understand extra about how this course of can work as well.
Singapore has been beckoning to traders ever since the value of properties in Singapore started sky rocketing just a few years again. Many businesses have their places of work in Singapore and prefer to own their own workplace area within the country once they decide to have a everlasting office. Rentals in Singapore in the corporate sector can make sense for some time until a business has discovered a agency footing. Finding Commercial Property Singapore takes a variety of time and effort but might be very rewarding in the long term.
is changing into a rising pattern among Singaporeans as the standard of living is increasing over time and more Singaporeans have abundance of capital to invest on properties. Investing in the personal properties in Singapore I would like to applaud you for arising with such a book which covers the secrets and techniques and tips of among the profitable Singapore property buyers. I believe many novice investors will profit quite a bit from studying and making use of some of the tips shared by the gurus." – Woo Chee Hoe Special bonus for consumers of Secrets of Singapore Property Gurus Actually, I can't consider one other resource on the market that teaches you all the points above about Singapore property at such a low value. Can you? Condominium For Sale (D09) – Yong An Park For Lease
In 12 months 2013, c ommercial retails, shoebox residences and mass market properties continued to be the celebrities of the property market. Models are snapped up in report time and at document breaking prices. Builders are having fun with overwhelming demand and patrons need more. We feel that these segments of the property market are booming is a repercussion of the property cooling measures no.6 and no. 7. With additional buyer's stamp responsibility imposed on residential properties, buyers change their focus to commercial and industrial properties. I imagine every property purchasers need their property funding to understand in value.
In chemistry, molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.[1] Because electrons are the fundamental constituents of matter involved in bonding, their involvement in bonding has been studied constantly by chemists. Electrons are shared among individual atoms in a molecule to form covalent chemical bonds. Generally, up to three bonds can form between atoms in a molecule. Single, or sigma covalent bonds result from the interaction between the nuclei of two discrete atoms; multiple bonds then can result due to the additional formation of pi bonds between overlapping orbitals of like symmetries.[2] Electrons in sigma bonds are located between the nuclei, while electrons in pi bonds are delocalized in regions above and below the nuclei.[3]
The spatial and energetic properties of electrons within atoms are fixed by quantum mechanics to form orbitals that contain these electrons.[4] While atomic orbitals contain electrons within a single atom, molecular orbitals, which surround a number of atoms in a molecule, contain valence electrons between atoms.[4] Molecular orbital theory, which was proposed in the early twentieth century, revolutionized the study of bonding by approximating the positions of bonded electrons—the molecular orbitals—as Linear Combinations of Atomic Orbitals (LCAO).[5] These approximations are made by applying the Density Functional Theory (DFT) and Hartree-Fock (HF) models to the Schrödinger equation.[6]
MO theory applies the wavelike behavior of electrons as predicted by quantum mechanics, in that electrons no longer deterministically are given defined coordinates, but rather are given probable locations according to the mathematical wavefunctions defining all the possible positions of the electrons. These wavefunctions, or electron eigenstates, quantitatively describe the atomic orbital basis in which an electron temporarily can reside. Molecular orbitals result from the mixing of these atomic orbitals. In this theory, each molecule has a set of molecular orbitals, in which it is assumed that the molecular orbital wave function ψj can be written as a simple weighted sum of the n constituent atomic orbitals χi, according to the following equation:[7]
Connection to MO diagrams and molecular geometry
Using molecular orbital theory, a molecular orbital diagram can be constructed that features the individual atomic basis orbitals and the molecular orbitals resulting from atomic interactions to form molecules. All atomic s, p, d and f orbitals are assigned relative electronic energies, and orbital interactions are predicted by the relative proximity in the energies of the individual atomic orbitals. The closer the atomic orbitals are in energy, the stronger and more likely their molecular orbital interaction is. Through qualitative molecular orbital analysis through a molecular orbital diagram, the chemist is able to display the resulting molecular orbitals stemming from atomic interaction based on their relative energies and assign bonding, nonbonding and/or antibonding character to each molecular orbital.[8] After these assignments are made, the chemist is able to identify the Highest Occupied Molecular Orbital (HOMO) that contains electrons and the Lowest Unoccupied Molecular Orbital (LUMO) that does not contain electrons. The difference in energy between these two frontier orbitals can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution.[9] The known geometries of all molecules and their (s,p,d,f) orbitals, as predicted by VSEPR theory, are used in conjunction with molecular orbital theory in determining whether select orbitals of an atomic basis are used for bonding, nonbonding, or antibonding interactions within a molecule. Bonding orbitals participate in strengthening the interaction between two atoms of a molecule; nonbonding orbitals exclusively are associated with one atom and usually represent a lone pair; antibonding orbitals participate in weakening the interaction between two atoms of a molecule.[10] Defining this interaction through symmetry adapted linear combinations (SALC’s), in which the geometry of valence orbital interaction is illustrated, allows the chemist to justify the assignment of a particular molecular orbital as either bonding, nonbonding or antibonding. SALC’s of a molecule can be drawn by knowing either the VSEPR geometry or point group symmetry of that molecule.[8]
Quantitative applications
One may determine cij coefficients numerically by substituting this equation into the Schrödinger equation and applying the variational principle. The variational principle is a mathematical technique used in quantum mechanics to build up the coefficients of each atomic orbital basis. A larger coefficient means that the orbital basis is composed more of that particular contributing atomic orbital—hence, the molecular orbital is best characterized by that type.[11] This method of quantifying orbital contribution as Linear Combinations of Atomic Orbitals is used in computational chemistry. An additional unitary transformation can be applied on the system to accelerate the convergence in some computational schemes. Molecular orbital theory was seen as a competitor to valence bond theory in the 1930s, before it was realized that the two methods are closely related and that when extended they become equivalent. Valence bond theory further describes the composition of each orbital in a molecule. Unlike MO theory, valence bond theory postulates that molecular orbitals are hybridized and contain a certain percentage of s, p and d character as determined by the number of bonds to the central atom (i.e. tetrahedral complexes feature four sp3 hybridized orbitals that contain 25% s character).[12] It similarly proposes that the strength of bonds increases with increasing overlap between atomic orbitals.[12]
History
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. Molecular orbital theory was developed, in the years after valence bond theory had been established (1927), primarily through the efforts of Friedrich Hund, Robert Mulliken, John C. Slater, and John Lennard-Jones.[13] MO theory was originally called the Hund-Mulliken theory.[14] The word orbital was introduced by Mulliken in 1932.[14] By 1933, the molecular orbital theory had been accepted as a valid and useful theory.[15] According to German physicist and physical chemist Erich Hückel, the first quantitative use of molecular orbital theory was the 1929 paper of Lennard-Jones.[16] The first accurate calculation of a molecular orbital wavefunction was that made by Charles Coulson in 1938 on the hydrogen molecule.[17] By 1950, molecular orbitals were completely defined as eigenfunctions (wave functions) of the self-consistent field Hamiltonian and it was at this point that molecular orbital theory became fully rigorous and consistent.[18] This rigorous approach is known as the Hartree–Fock method for molecules although it had its origins in calculations on atoms. In calculations on molecules, the molecular orbitals are expanded in terms of an atomic orbital basis set, leading to the Roothaan equations.[19] This led to the development of many ab initio quantum chemistry methods. In parallel, molecular orbital theory was applied in a more approximate manner using some empirically derived parameters in methods now known as semi-empirical quantum chemistry methods.[19]
Application to ligand field theory
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. Ligand field theory resulted from combining the principles laid out in molecular orbital theory and crystal field theory, which describes the loss of degeneracy of frontier orbitals in transition metal complexes. Griffith and Orgel[9] championed ligand field theory as a more accurate description of transition metal coordinated compounds. They used the electrostatic principles established in crystal field theory to describe transition metal ions in solutions, and they used molecular orbital theory to explain the differences in transition metal complex interactions. In their paper, they propose that the chief consequence of differences in colors produced by transition metal complexes in solution is incomplete d orbital subshells.[9] That is, the d orbitals of transition metals unoccupied by electrons participate in the bonding that influences the colors they emit in solution. Through ligand field theory, the connection between d orbital energy of transition metal complexes and the character of ligands bound to the transition metal was established. Based on the geometric symmetry between the various d orbitals (dz2, dx2-y2, dxz, dyz, dxy) of the metal and its associated ligands, the relative energies of the d orbitals could be qualitatively and quantitatively assessed. Ligand field theory, therefore, explains how transition metal complexes adjust structurally to reduce degeneracy among d orbital energies. This phenomenon is known as the Jahn-Teller effect. Because d orbital energies are affected differently when surrounded by a field of neighboring ligands, as determined by SALC’s, they separately are raised or lowered in energy based on the intensity that their geometries permit them to interact with the ligands.[9] Thus, ligand field theory allows the chemist to determine d orbital energy splittings in transition metal complexes based on the VSEPR-assigned geometries of these compounds. For instance, octahedral complexes are known from SALC’s to feature the greatest interaction between the dz2 frontier orbital and the ligands. Relative interaction intensities between the d orbitals and ligands for other geometries, such as square planar and tetrahedral, also are known.[9]
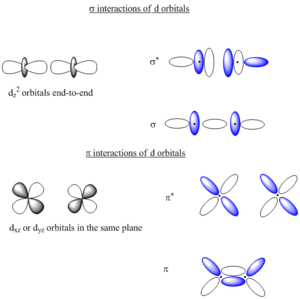
The potential of the d orbitals to interact with the ligands affects the relative energies between them.[9] Due to the toroidal shape of the dz2 orbital, it is capable of interacting with ligands in both the axial and equatorial orientations, resulting in its energy being comparatively raised relative to the other d orbitals due to the repulsive interaction with the ligands. Furthermore, in tetrahedral and octahedral complexes, the stronger overall interaction of the dz2 and dx2-y2 orbitals with the ligands induces the separation of these two d orbitals from the dxz, dyz, and dxy orbtials. This results in d orbital splitting into a doubly degenerate (eg) state and a triply degenerate (t2g) state.[9] The energy splitting of the two d orbital states is a predictor of electron spin state, stability, and luminescent color emitted by the complex in solution. Electrostatic theory can be used to predict electronic transitions between the ground-state t2g and excited state eg energy levels.[9] Complexes in which electrons fill the t2g orbitals prior to filling the eg orbitals are referred to as low-spin complexes; conversely, complexes in which electrons will fill the eg orbitals prior to completely filling the t2g orbitals, thereby maximizing the number of electron parallel spins, are referred to as high-spin complexes.[8]
Overview
Molecular orbital (MO) theory uses a linear combination of atomic orbitals (LCAO) to represent molecular orbitals resulting from bonds between atoms.[4] These are often divided into bonding orbitals, anti-bonding orbitals, and non-bonding orbitals. The Schrödinger equation—an application of the wave equation to quantum mechanics used to build up the orbital bases of an atom or molecule—can be solved in three-dimensions to determine the shape of each molecular orbital.[4] A molecular orbital is a Schrödinger orbital that includes two or more nuclei. If this orbital is of the type in which the electron(s) in the orbital have a higher probability of being between nuclei than elsewhere, the orbital will be a bonding orbital, and will tend to hold the nuclei together.[8] A bonding orbital is of lower energy than the two contributing atomic bases, and the percentage composition of each atomic orbital to the molecular orbital is determined by molecular orbital theory. For bonding orbitals, the atomic orbital of lower energy contributes more to the molecular orbital, causing the molecular orbital to more closely resemble it.[20] If the electrons tend to be present in a molecular orbital in which they spend more time elsewhere than between the nuclei, the orbital will function as an anti-bonding orbital and will actually weaken the bond. Conversely, an antibonding orbital will more closely resemble the higher energy atomic basis.[8] Electrons in non-bonding orbitals tend to be in deep orbitals (nearly atomic orbitals) associated almost entirely with one nucleus or the other, and thus they spend equal time within as they do between nuclei. These electrons neither contribute to nor detract from bond strength and thus are best described as lone pairs.[8]
Molecular orbitals are further divided according to the types of atomic orbitals combining to form a bond. These orbitals are results of electron-nucleus interactions that are caused by the fundamental force of electromagnetism—the attraction between the positively charged nucleus and negatively charged electron.[21]
Quantum mechanics stipulates that an electron’s position is confined solely to the atomic orbital basis, characterized by s, p, d and f subshells with unique shielding parameters. Chemical substances will form a bond if their orbitals become lower in energy when they interact with each other. This decrease in energy may stem from pi backbonding of a ligand to a metal, which can stabilize a metal’s electron pair through delocalization.[8] Thus, bonding molecular orbitals have greater electron density than their component atomic orbitals. Different chemical bonds are distinguished that differ by electron configuration (electron cloud shape) and by energy levels. All atomic orbitals are characterized by a principal atomic number n, or energy level, an azimuthal quantum number l distinguishing s, p, d and f subshells, and a magnetic quantum number m defined as the range –l<m<l. In addition, all electrons are placed into orbitals and are assigned appropriate spin according to the Aufbau principle, Hund’s rule and the Pauli exclusion principle.[8]
Molecular orbital diagrams are how chemists analyze the electron configuration of a molecule. They are constructed as qualitative descriptions of bonding within molecules by invoking Molecular Orbital Theory. While VSEPR and valence bond theory are applied to predict the structures of molecules,[10] MO theory is used to determine orbital interaction and valence electron configuration within the molecular orbitals of a molecule. MO diagrams incorporate the wavelike behavior of electrons as postulated by MO theory.[10] They are used to predict the shape of a molecule, as well as the energy, length and angle of each bond.[10] Upon constructing an MO diagram, the following stipulations must be met:[10]
- Orbitals are built up along the y-axis from lowest to highest energy.
- Atomic orbitals of the individual atoms are drawn to the left and right of the molecular orbitals, and are traced to each MO they contribute to.
- Overlapping atomic orbitals contribute to molecular orbitals that possess σ, π, and/or nonbonding character.
- Valence electrons from the atomic orbitals are assigned to molecular orbitals by the Pauli Exclusion Principle.
A complete MO diagram therefore reveals the transformation of all the atomic orbitals into their resulting molecular orbital descriptions. MO diagrams are filled by first determining the number of valence electrons contributed by all the atoms of a molecule, and then evaluating the strength of the orbital interactions by the degree of overlap. Because σ bonds feature greater overlap than π bonds, which are usually long-range, σ and σ* bonding and antibonding orbitals, respectively, feature greater energy splitting (separation) than π and π* orbitals.[10]
MO theory provides a global, delocalized perspective on chemical bonding. In MO theory, any electron in a molecule may be found anywhere in the molecule, since quantum conditions allow electrons to travel under the influence of an arbitrarily large number of nuclei, as long as they are in eigenstates permitted by certain quantum rules. Thus, when excited with the requisite amount of energy through high-frequency light or other means, electrons can transition to higher-energy—even antibonding—molecular orbitals.[4] For instance, in the simple case of a hydrogen diatomic molecule, promotion of a single electron from a bonding orbital to an antibonding orbital can occur under UV radiation. This promotion weakens the bond between the two hydrogen atoms and can lead to photodissociation—the breaking of a chemical bond due to the absorption of light.[22]
Although in MO theory some molecular orbitals may hold electrons that are more localized between specific pairs of molecular atoms, other orbitals may hold electrons that are spread more uniformly over the molecule. Thus, overall, bonding is far more delocalized in MO theory, which makes it more applicable to resonant molecules that have equivalent non-integer bond orders than valence bond (VB) theory.[23] For instance, MO theory is used to explain the resonant intermediate bond character of NO3-, in which the N-O bonds have a mix of single and double bond character. This makes MO theory more useful for the description of extended systems.
An example is the MO description of benzene, Template:Chem, which is an aromatic hexagonal ring of six carbon atoms and three double bonds. In this molecule, 24 of the 30 total valence bonding electrons—24 coming from carbon atoms and 6 coming from hydrogen atoms—are located in 12 σ (sigma) bonding orbitals, which are located mostly between pairs of atoms (C-C or C-H), similarly to the electrons in the valence bond description.[6] However, in benzene the remaining six bonding electrons are located in three π (pi) molecular bonding orbitals that are delocalized around the ring.[6] Two of these electrons are in an MO that has equal resonant contributions from all six atoms. The other four electrons are in orbitals with vertical nodes at right angles to each other. As in the VB theory, all of these six delocalized π electrons reside in a larger space that exists above and below the ring plane. All carbon-carbon bonds in benzene are chemically equivalent. In MO theory this is a direct consequence of the fact that the three molecular π orbitals combine and evenly spread the extra six electrons over six carbon atoms.[4]

In molecules such as methane, Template:Chem, the eight valence electrons are found in four MOs that are spread out over all five atoms. However, it is possible to approximate the MOs with four localized orbitals similar in shape to the sp3 hybrid orbitals predicted by VB theory.[12] Linus Pauling, in 1931, hybridized the carbon 2s and 2p orbitals so that they pointed directly at the hydrogen 1s basis functions and featured maximal overlap.[12] This is often adequate for σ bonds, but is not possible for the π orbitals. However, the delocalized MO description is more appropriate for ionization and spectroscopic predictions.[12] When methane is ionized, a single electron is taken from the MO, which surrounds the whole molecule, weakening all four bonds equally. VB theory would predict that one electron is removed from an sp3 orbital, resulting in the need for resonance between four valence bond structures, each of which has a single one-electron bond and three two-electron bonds. Thus, while the VB model predicts only one ionization energy for methane, there are two ionization energies from the lowest molecular orbitals as predicted by MO theory.[12]
As in benzene, in substances such as beta carotene, chlorophyll, or heme, some electrons in the π orbitals are spread out in molecular orbitals over long distances in a molecule, resulting in light absorption in lower energies (the visible spectrum), which accounts for the characteristic colours of these substances.[9] This and other spectroscopic data for molecules are better explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching.[8] The same MO principles also more naturally explain some electrical phenomena, such as high electrical conductivity in the planar direction of the hexagonal atomic sheets that exist in graphite. This results from continuous band overlap of half-filled p orbitals.[24] In MO theory, "resonance" is a natural consequence of symmetry. For example, in graphite, as in benzene, it is not necessary to invoke the sp2 hybridization and resonance of VB theory, in order to explain electrical conduction. Instead, MO theory recognizes that some electrons in the graphite atomic sheets are completely delocalized over arbitrary distances, and reside in very large molecular orbitals that cover an entire graphite sheet, and some electrons are thus as free to move and therefore conduct electricity in the sheet plane, as if they resided in a metal.[25]
See also
Template:Col-begin Template:Col-break
- Ab initio quantum chemistry methods
- Atomic orbital
- Configuration interaction
- Coupled cluster
- Hartree–Fock method
- Molecular orbital
- Molecular orbital diagram
- Møller–Plesset perturbation theory
- Quantum chemistry computer programs
- Semi-empirical quantum chemistry methods
- cis effect
- Addition to pi ligands
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- Molecular Orbital Theory - Purdue University
- Molecular Orbital Theory - Sparknotes
- Molecular Orbital Theory - Mark Bishop's Chemistry Site
- Introduction to MO Theory - Queen Mary, London University
- Molecular Orbital Theory - a related terms table
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ “Sigma and Pi Bonds.” 11/8/13. <http://www.kentchemistry.com/links/bonding/sigmapi.htm>
- ↑ “Sigma and Pi Bonds.” 11/8/13. <http://www.kentchemistry.com/links/bonding/sigmapi.htm>
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Introduction to Molecular Orbital Theory - Imperial College London
- ↑ “Linear combination of atomic orbitals.” 11/8/13. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_14/node1.html>.
- ↑ 6.0 6.1 6.2 “Molecular Orbitals of Benzene.” 11/8/13. < http://csi.chemie.tu-darmstadt.de/ak/immel/script/redirect.cgi?filename=http://csi.chemie.tu-darmstadt.de/ak/immel/tutorials/orbitals/molecular/benzene.html>.
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 Miessler and Tarr (2013), Inorganic Chemistry, 5th ed, 117-165, 475-534.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 Griffith, J.S. and L.E. Orgel. Ligand Field Theory. Q. Rev. Chem. Soc. 1957, 11, 381-383. <http://pubs.rsc.org/en/content/articlelanding/1957/qr/qr9571100381#!divAbstract>.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 “How to Build Molecular Orbitals.” UC Davis ChemWiki. 11/8/13. <http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/Molecular_Orbital_Theory/How_to_Build_Molecular_Orbitals>.
- ↑ “Linear combination of atomic orbitals.” 11/8/13. <http://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_14/node1.html>.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 “A Molecular Orbital Approach to Bonding in Methane.” 11/8/13. <http://www.users.csbsju.edu/~frioux/h2bond/MethaneMOBonding.pdf>
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 14.0 14.1 Template:Cite press release
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Many property agents need to declare for the PIC grant in Singapore. However, not all of them know find out how to do the correct process for getting this PIC scheme from the IRAS. There are a number of steps that you need to do before your software can be approved.
Naturally, you will have to pay a safety deposit and that is usually one month rent for annually of the settlement. That is the place your good religion deposit will likely be taken into account and will kind part or all of your security deposit. Anticipate to have a proportionate amount deducted out of your deposit if something is discovered to be damaged if you move out. It's best to you'll want to test the inventory drawn up by the owner, which can detail all objects in the property and their condition. If you happen to fail to notice any harm not already mentioned within the inventory before transferring in, you danger having to pay for it yourself.
In case you are in search of an actual estate or Singapore property agent on-line, you simply should belief your intuition. It's because you do not know which agent is nice and which agent will not be. Carry out research on several brokers by looking out the internet. As soon as if you end up positive that a selected agent is dependable and reliable, you can choose to utilize his partnerise in finding you a home in Singapore. Most of the time, a property agent is taken into account to be good if he or she locations the contact data on his website. This may mean that the agent does not mind you calling them and asking them any questions relating to new properties in singapore in Singapore. After chatting with them you too can see them in their office after taking an appointment.
Have handed an trade examination i.e Widespread Examination for House Brokers (CEHA) or Actual Property Agency (REA) examination, or equal; Exclusive brokers are extra keen to share listing information thus making certain the widest doable coverage inside the real estate community via Multiple Listings and Networking. Accepting a severe provide is simpler since your agent is totally conscious of all advertising activity related with your property. This reduces your having to check with a number of agents for some other offers. Price control is easily achieved. Paint work in good restore-discuss with your Property Marketing consultant if main works are still to be done. Softening in residential property prices proceed, led by 2.8 per cent decline within the index for Remainder of Central Region
Once you place down the one per cent choice price to carry down a non-public property, it's important to accept its situation as it is whenever you move in – faulty air-con, choked rest room and all. Get round this by asking your agent to incorporate a ultimate inspection clause within the possibility-to-buy letter. HDB flat patrons routinely take pleasure in this security net. "There's a ultimate inspection of the property two days before the completion of all HDB transactions. If the air-con is defective, you can request the seller to repair it," says Kelvin.
15.6.1 As the agent is an intermediary, generally, as soon as the principal and third party are introduced right into a contractual relationship, the agent drops out of the image, subject to any problems with remuneration or indemnification that he could have against the principal, and extra exceptionally, against the third occasion. Generally, agents are entitled to be indemnified for all liabilities reasonably incurred within the execution of the brokers´ authority.
To achieve the very best outcomes, you must be always updated on market situations, including past transaction information and reliable projections. You could review and examine comparable homes that are currently available in the market, especially these which have been sold or not bought up to now six months. You'll be able to see a pattern of such report by clicking here It's essential to defend yourself in opposition to unscrupulous patrons. They are often very skilled in using highly unethical and manipulative techniques to try and lure you into a lure. That you must also protect your self, your loved ones, and personal belongings as you'll be serving many strangers in your home. Sign a listing itemizing of all of the objects provided by the proprietor, together with their situation. HSR Prime Recruiter 2010 - ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ 19.0 19.1 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ A bonding orbital is of lower energy than the two contributing atomic bases, and the percentage composition of each atomic orbital to the molecular orbital is determined by molecular orbital theory. For bonding orbitals, the atomic orbital of lower energy contributes more to the molecular orbital, causing the molecular orbital to more closely resemble it.
- ↑ “The Four Fundamental Forces.” Oracle ThinkQuest. 11/8/13. <http://library.thinkquest.org/27930/forces.htm>.
- ↑ “Photodissociation Dynamics.” Kable Group. University of Sydney. 11/8/13. <http://sydney.edu.au/science/chemistry/~kable_s/projects/dynamics/>.
- ↑ “Molecular Structure and Bonding: Bonding Theories.” 11/8/13. <http://www.chem.latech.edu/~upali/chem281/notes/Ch2-MO-Theory.pdf>.
- ↑ “Benzene and Graphite.” 11/8/13. <http://www.seas.upenn.edu/~chem101/sschem/conduction.html#graphite>.
- ↑ “Benzene and Graphite.” 11/8/13. <http://www.seas.upenn.edu/~chem101/sschem/conduction.html#graphite>.
