Curie constant: Difference between revisions
en>Helpful Pixie Bot m ISBNs (Build KE) |
en>Maurice Carbonaro m Hyperlinked "(...) SI units (...)" to the "International System of Units" article |
||
| Line 1: | Line 1: | ||
{{Distinguish2|[[Adsorption]]}} | |||
[[File:Absorber.svg|thumb|250px|Laboratory absorber. '''1a)''': CO<sub>2</sub> inlet; '''1b)''': H<sub>2</sub>O inlet; '''2)''': outlet; '''3)''': absorption column; '''4)''': packing.]] | |||
In [[chemistry]], '''absorption''' is a physical or chemical [[phenomenon]] or a [[Process (science)|process]] in which [[atom]]s, [[molecules]], or [[ion]]s enter some bulk phase – [[gas]], [[liquid]], or [[solid]] material. This is a different process from [[adsorption]], since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more general term is ''[[sorption]]'', which covers absorption, [[adsorption]], and [[ion exchange]]. Absorption is a condition in which something takes in another substance.<ref name=Organic-chemistry/> | |||
In many processes important in technology, the chemical absorption is used in place of the physical process, e.g., absorption of carbon dioxide by sodium hydroxide – such acid-base processes do not follow the Nernst partition law. | |||
For some examples of this effect, see [[liquid-liquid extraction]]. It is possible to extract from one [[liquid]] phase to another a [[Solution|solute]] without a chemical reaction. Examples of such solutes are [[noble gases]] and [[osmium tetroxide]].<ref name=Organic-chemistry> | |||
{{cite book|last = McMurry|first = John|title = Fundamentals of Organic Chemistry | |||
|edition = Fifth|publisher = Agnus McDonald|year = 2003 | |||
|pages = 409|isbn = 0-534-39573-2}}</ref> | |||
The process of absorption means that a substance captures and transforms energy. The absorbent distributes the material it captures throughout whole and adsorbent only distributes it through the surface. The reddish color of copper is an example of this process because it is caused due to its absorption of blue light.<ref>Senese, F. (1997-2010) General Chemistry Online. Obtained on December 1, 2012 from http://antoine.frostburg.edu/chem/senese/101/glossary/a.shtml</ref> | |||
==Equation== | |||
If absorption is a physical process not accompanied by any other physical or chemical process, it usually follows the '''[[distribution law|Nernst distribution law]]''': | |||
:"the ratio of concentrations of some solute species in two bulk phases in contact is constant for a given solute and bulk phases": | |||
:: <math>\frac{[x]_{1}}{[x]_{2}} = \text{constant} = K_{N(x,12)}</math> | |||
The value of constant K<sub>N</sub> depends on temperature and is called '''[[partition coefficient]]'''. This equation is valid if concentrations are not too large and if the species "x" does not change its form in any of the two phases "1" or "2". If such molecule undergoes association or [[Dissociation (chemistry)|dissociation]] then this equation still describes the equilibrium between "x" in both phases, but only for the same form – concentrations of all remaining forms must be calculated by taking into account all the other equilibria.<ref name=Organic-chemistry/> | |||
In the case of gas absorption, one may calculate its concentration by using, e.g., the [[Ideal gas law]], c = p/RT. In alternative fashion, one may use [[partial pressure]]s instead of concentrations. | |||
==Types of absorption== | |||
Absorption is a process that may be chemical (reactive) or physical (non-reactive). | |||
=== Physical absorption === | |||
Physical absorption or non-reactive absorption is made between two phases of matter: a liquid absorbs a gas, or a solid absorbs a liquid. | |||
When a liquid solvent absorbs a gas mixture or part of it, a mass of gas moves into the liquid. For example, water may absorb oxygen from the air. This mass transfer takes place at the interface between the liquid and the gas, at a rate depending on both the gas and the liquid. This type of absorption depends on the solubility of gases, the pressure and the temperature.<ref>(n.a.) (December 4, 2010) Absorption (Chemistry). Obtained on December 1, 2012 from http://en.citizendium.org/wiki/Absorption_(chemistry)</ref> The rate and amount of absorption also depend on the surface area of the interface and its duration in time. For example, when the water is finely divided and mixed with air, as may happen in a waterfall or a strong ocean surf, the water absorbs more oxygen. | |||
When a solid absorbs a liquid mixture or part of it, a mass of liquid moves into the solid. For example, a [[Pottery|clay pot]] used to store water may absorb some of the water. This mass transfer takes place at the interface between the solid and the liquid, at a rate depending on both the solid and the liquid. For example, pots made from certain [[Pottery#Clay bodies and mineral contents|clays]] are more absorbent than others. | |||
=== Chemical absorption === | |||
Chemical absorption or reactive absorption is a chemical reaction between the absorbed and the absorbing substances. Sometimes it combines with physical absorption. This type of absorption depends upon the [[stoichiometry]] of the reaction and the concentration of its reactants.<ref>{{cite journal|last=Leiviskä|first=Tiina|author2=Gehör, Seppo|author3=Eijärvi, Erkki|author4=Sarpola, Arja|author5=Tanskanen, Juha|title=Characteristics and potential applications of coarse clay fractions from Puolanka, Finland|journal=Central European Journal of Engineering|date=10 April 2012|volume=2|issue=2|pages=239–247|doi=10.2478/s13531-011-0067-9}}</ref> | |||
==References== | |||
{{Reflist|2}} | |||
[[Category:Physical chemistry]] | |||
Revision as of 10:56, 23 November 2013
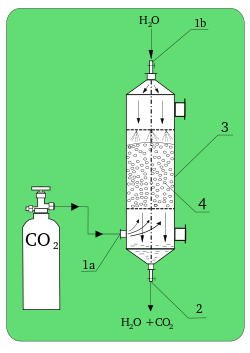
In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules, or ions enter some bulk phase – gas, liquid, or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more general term is sorption, which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance.[1]
In many processes important in technology, the chemical absorption is used in place of the physical process, e.g., absorption of carbon dioxide by sodium hydroxide – such acid-base processes do not follow the Nernst partition law.
For some examples of this effect, see liquid-liquid extraction. It is possible to extract from one liquid phase to another a solute without a chemical reaction. Examples of such solutes are noble gases and osmium tetroxide.[1]
The process of absorption means that a substance captures and transforms energy. The absorbent distributes the material it captures throughout whole and adsorbent only distributes it through the surface. The reddish color of copper is an example of this process because it is caused due to its absorption of blue light.[2]
Equation
If absorption is a physical process not accompanied by any other physical or chemical process, it usually follows the Nernst distribution law:
- "the ratio of concentrations of some solute species in two bulk phases in contact is constant for a given solute and bulk phases":
The value of constant KN depends on temperature and is called partition coefficient. This equation is valid if concentrations are not too large and if the species "x" does not change its form in any of the two phases "1" or "2". If such molecule undergoes association or dissociation then this equation still describes the equilibrium between "x" in both phases, but only for the same form – concentrations of all remaining forms must be calculated by taking into account all the other equilibria.[1]
In the case of gas absorption, one may calculate its concentration by using, e.g., the Ideal gas law, c = p/RT. In alternative fashion, one may use partial pressures instead of concentrations.
Types of absorption
Absorption is a process that may be chemical (reactive) or physical (non-reactive).
Physical absorption
Physical absorption or non-reactive absorption is made between two phases of matter: a liquid absorbs a gas, or a solid absorbs a liquid.
When a liquid solvent absorbs a gas mixture or part of it, a mass of gas moves into the liquid. For example, water may absorb oxygen from the air. This mass transfer takes place at the interface between the liquid and the gas, at a rate depending on both the gas and the liquid. This type of absorption depends on the solubility of gases, the pressure and the temperature.[3] The rate and amount of absorption also depend on the surface area of the interface and its duration in time. For example, when the water is finely divided and mixed with air, as may happen in a waterfall or a strong ocean surf, the water absorbs more oxygen.
When a solid absorbs a liquid mixture or part of it, a mass of liquid moves into the solid. For example, a clay pot used to store water may absorb some of the water. This mass transfer takes place at the interface between the solid and the liquid, at a rate depending on both the solid and the liquid. For example, pots made from certain clays are more absorbent than others.
Chemical absorption
Chemical absorption or reactive absorption is a chemical reaction between the absorbed and the absorbing substances. Sometimes it combines with physical absorption. This type of absorption depends upon the stoichiometry of the reaction and the concentration of its reactants.[4]
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- ↑ 1.0 1.1 1.2
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Senese, F. (1997-2010) General Chemistry Online. Obtained on December 1, 2012 from http://antoine.frostburg.edu/chem/senese/101/glossary/a.shtml
- ↑ (n.a.) (December 4, 2010) Absorption (Chemistry). Obtained on December 1, 2012 from http://en.citizendium.org/wiki/Absorption_(chemistry)
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang
![{\displaystyle {\frac {[x]_{1}}{[x]_{2}}}={\text{constant}}=K_{N(x,12)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e0ec1ce71d0ce27734b33e4d1dfae04be91ecf13)