Main Page: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{| border="1" style=" | <!-- To obtain a blank version of this page, type {{chembox supplement}} and save the page --> | ||
| | |||
This page provides supplementary chemical data on [[ammonia]]. | |||
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
! {{chembox header}} | Molecular structure | |||
|- | |||
| [[Point group]] | |||
| C<sub>3v</sub> | |||
|- | |||
| [[Bond length]] | |||
| 101.7 [[picometre|pm]] (N–H) | |||
|- | |||
| [[Bond angle]] | |||
| 107.8° (H–N–H) | |||
|- | |||
| [[Bond strength (chemistry)|Bond strength]] | |||
| 435 kJ/mol (H–NH<sub>2</sub>) | |||
|- | |||
! {{chembox header}} | Crystal data | |||
|- | |||
| [[Crystal structure]] <!-- omit if not a solid --> | |||
| ? <!-- e.g. [[triclinic]], [[monoclinic]], [[orthorhombic]], [[hexagonal]], [[rhombohedral|trigonal]], [[tetragonal]], [[cubic]], and mention "close packed" or similar. You may also cite what class it belongs to, e.g. [[Cadmium chloride#Crystal structure|CdCl<sub>2</sub>]] --> | |||
|- | |||
! {{chembox header}} | Properties | |||
|- | |- | ||
|[[ | | [[Dipole#Molecular dipoles|Dipole moment]] | ||
| 1.46 [[Debye|D]] | |||
|- | |- | ||
| [[ | | [[Dielectric constant]] | ||
| | | 22 ε<sub>0</sub> at 239 K | ||
|- | |- | ||
| [[ | | [[Magnetic susceptibility]] | ||
| | | diamagnetic | ||
|- | |- | ||
| [[ | | [[Acid dissociation constant|Acidity of NH<sub>4</sub><sup>+</sup>]] ([[Acid dissociation constant|p''K''<sub>a</sub>]]) | ||
| < | | 9.25 | ||
|} | |} | ||
== Thermodynamic properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Phase behavior | |||
|- | |||
| [[Triple point]] | |||
| 195.4 K (–77.75 °C), 6.060 k[[Pascal (unit)|Pa]] | |||
|- | |||
| [[Critical point (thermodynamics)|Critical point]] | |||
| 405.5 K (132.3 °C), 11.300 M[[Pascal (unit)|Pa]] | |||
|- | |||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]], Δ<sub>fus</sub>''H''<sup><s>o</s></sup> | |||
| +5.653 kJ/mol | |||
|- | |||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]], Δ<sub>fus</sub>''S''<sup><s>o</s></sup> | |||
| +28.93 J/(mol·K) | |||
|- | |||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]], Δ<sub>vap</sub>''H''<sup><s>o</s></sup> | |||
| +23.35 kJ/mol at [[boiling point|BP]] of –33.4 °C | |||
|- | |||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]], Δ<sub>vap</sub>''S''<sup><s>o</s></sup> | |||
| +97.41 J/(mol·K) at [[boiling point|BP]] of –33.4 °C | |||
|- | |||
! {{chembox header}} | Solid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| ? J/(mol K) | |||
|- | |||
! {{chembox header}} | Liquid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| ? kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>liquid</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 80.80 J/(mol K) | |||
|- | |||
! {{chembox header}} | Gas properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>gas</sub> | |||
| −45.92 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>gas</sub> | |||
| 192.77 J/(mol K) | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 35.06 J/(mol K) | |||
|- | |||
| [[Heat capacity ratio]], ''γ''<br>at 15°C | |||
| 1.310 | |||
|- | |||
| [[Van der Waals equation|van der Waals' constants]] | |||
| a = 422.5 liter<sup>2</sup> k[[Pascal (unit)|Pa]] / mole<sup>2</sup><br> b = 0.03707 liter / mole | |||
|- | |||
|} | |||
==Vapor-Liquid Equilibrium Data== | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| {{chembox header}} | '''P in mm Hg''' || 1 || 10 || 40 || 100 || 400 || 760 || 1520 || 3800 || 7600 || 15600 || 30400 || 45600 | |||
|- | |||
| {{chembox header}} | '''T in °C''' || –109.1<sub>(s)</sub> || –91.9<sub>(s)</sub> || –79.2<sub>(s)</sub> || –68.4 || –45.4 || –33.6 || –18.7 || 4.7 || 25.7 || 50.1 || 78.9 || 98.3 | |||
|} | |||
Table data (above) obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. | |||
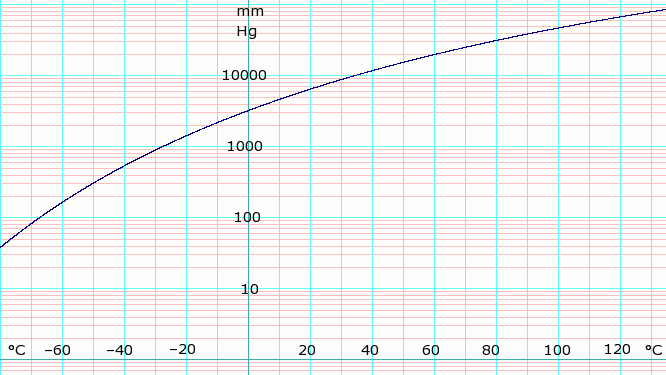
: | [[Image:LogAmmoniaVaporPressure.png|thumb|666px|left|'''log<sub>10</sub> of anydrous Ammonia vapor pressure.''' Uses formula shown below.]]{{Clear}} | ||
Vapor pressure formula for ammonia:<ref>Lange's Handbook of Chemistry, 10th ed. page 1436</ref> | |||
:::log<sub>10</sub>(P) = A – B / (T – C) | |||
where ''P'' is pressure in k[[pascal (unit)|Pa]] and ''T'' is temperature in [[kelvin]]s | |||
:::A = 6.67956; B = 1002.711; C = 25.215 for T = 190 K through 333 K | |||
:: | |||
== | {| border="0" cellpadding="0" cellspacing="0" | ||
|----- | |||
| valign="top" width="60%" | | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: {{{bgc|white}}}; border-collapse: collapse; border-color: {{{bc|#C0C090}}};" | |||
! {{chembox header}} colspan="5" | Vapor over Anhydrous Ammonia<ref>Lange's Handbook of Chemistry, 10th ed. page 1451 and 1468</ref> | |||
|- | |||
| bgcolor="E0E0E0" align="center" | Temp. | |||
| bgcolor="E0E0E0" align="center" | Pressure | |||
| bgcolor="E0E0E0" align="center" | ''ρ'' of liquid | |||
| bgcolor="E0E0E0" align="center" | ''ρ'' of vapor | |||
| bgcolor="E0E0E0" align="center" | ''Δ''<sub>vap</sub>''H'' | |||
|- | |||
| –78 °C || 5.90 k[[Pascal (unit)|Pa]] | |||
| || | |||
|- | |||
| –75 °C || 7.93 kPa | |||
| 0.73094 g/cm<sup>3</sup> | |||
| 7.8241×10<sup>−5</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| –70 °C || 10.92 kPa | |||
| 0.72527 g/cm<sup>3</sup> | |||
| 1.1141×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| –65 °C || 15.61 kPa | |||
| 0.71953 g/cm<sup>3</sup> | |||
| 1.5552×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| –60 °C || 21.90 kPa | |||
| 0.71378 g/cm<sup>3</sup> | |||
| 2.1321×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| –55 °C || 30.16 kPa | |||
| 0.70791 g/cm<sup>3</sup> | |||
| 2.8596×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| –50 °C || 40.87 kPa | |||
| 0.70200 g/cm<sup>3</sup> | |||
| 3.8158×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| 1417 J/g | |||
|- | |||
| –45 °C || 54.54 kPa | |||
| 0.69604 g/cm<sup>3</sup> | |||
| 4.9940×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| 1404 J/g | |||
|- | |||
| –40 °C || 71.77 kPa | |||
| 0.68999 g/cm<sup>3</sup> | |||
| 6.4508×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| 1390 J/g | |||
|- | |||
| –35 °C || 93.19 kPa | |||
| 0.68385 g/cm<sup>3</sup> | |||
| 8.2318×10<sup>−4</sup> g/cm<sup>3</sup> | |||
| 1375 J/g | |||
|- | |||
| –30 °C || 119.6 kPa | |||
| 0.67764 g/cm<sup>3</sup> | |||
| 1.0386×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1361 J/g | |||
|- | |||
| –25 °C || 151.6 kPa | |||
| 0.67137 g/cm<sup>3</sup> | |||
| 1.2969×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1345 J/g | |||
|- | |||
| –20 °C || 190.2 kPa | |||
| 0.66503 g/cm<sup>3</sup> | |||
| 1.6039×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1330 J/g | |||
|- | |||
| –15 °C || 236.3 kPa | |||
| 0.65854 g/cm<sup>3</sup> | |||
| 1.9659×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1314 J/g | |||
|- | |||
| –10 °C || 290.8 kPa | |||
| 0.65198 g/cm<sup>3</sup> | |||
| 2.3874×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1297 J/g | |||
|- | |||
| –5 °C || 354.8 kPa | |||
| 0.64533 g/cm<sup>3</sup> | |||
| 2.8827×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1280 J/g | |||
|- | |||
| 0 °C || 429.4 kPa | |||
| 0.63857 g/cm<sup>3</sup> | |||
| 3.4528×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1263 J/g | |||
|- | |||
| 5 °C || 515.7 kPa | |||
| 0.63167 g/cm<sup>3</sup> | |||
| 4.1086×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1245 J/g | |||
|- | |||
| 10 °C || 614.9 kPa | |||
| 0.62469 g/cm<sup>3</sup> | |||
| 4.8593×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1226 J/g | |||
|- | |||
| 15 °C || 728.3 kPa | |||
| 0.61755 g/cm<sup>3</sup> | |||
| 5.7153×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1207 J/g | |||
|- | |||
| 20 °C || 857.1 kPa | |||
| 0.61028 g/cm<sup>3</sup> | |||
| 6.6876×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1187 J/g | |||
|- | |||
| 25 °C || 1003 kPa | |||
| 0.60285 g/cm<sup>3</sup> | |||
| 7.7882×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1167 J/g | |||
|- | |||
| 30 °C || 1166 kPa | |||
| 0.59524 g/cm<sup>3</sup> | |||
| 9.0310×10<sup>−3</sup> g/cm<sup>3</sup> | |||
| 1146 J/g | |||
|- | |||
| 35 °C || 1350 kPa | |||
| 0.58816 g/cm<sup>3</sup> | |||
| 1.0431×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| 1124 J/g | |||
|- | |||
| 40 °C || 1554 kPa | |||
| 0.57948 g/cm<sup>3</sup> | |||
| 1.2006×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| 1101 J/g | |||
|- | |||
| 45 °C || 1781 kPa | |||
| 0.57130 g/cm<sup>3</sup> | |||
| 1.3775×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| 1083 J/g | |||
|- | |||
| 50 °C || 2032 kPa | |||
| 0.56287 g/cm<sup>3</sup> | |||
| 1.5761×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| 1052 J/g | |||
|- | |||
| 55 °C || 2310 kPa | |||
| 0.55420 g/cm<sup>3</sup> || | |||
|| | |||
|- | |||
| 60 °C || 2613 kPa | |||
| 0.54523 g/cm<sup>3</sup> | |||
| 2.05×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| 65 °C || 2947 kPa | |||
| 0.53596 g/cm<sup>3</sup> || | |||
|| | |||
|- | |||
| 70 °C || 3312 kPa | |||
| 0.52632 g/cm<sup>3</sup> | |||
| 2.65×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| 75 °C || 3711 kPa | |||
| 0.51626 g/cm<sup>3</sup> || | |||
|| | |||
|- | |||
| 80 °C || 4144 kPa | |||
| 0.50571 g/cm<sup>3</sup> | |||
| 3.41×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| 85 °C || 4614 kPa | |||
| 0.49463 g/cm<sup>3</sup> || | |||
|| | |||
|- | |||
| 90 °C || 5123 kPa | |||
| 0.48290 g/cm<sup>3</sup> | |||
| 4.39×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
| 95 °C || 5672 kPa | |||
| 0.47041 g/cm<sup>3</sup> || | |||
|| | |||
|- | |||
| 100 °C || 6264 kPa | |||
| 0.45693 g/cm<sup>3</sup> | |||
| 5.68×10<sup>−2</sup> g/cm<sup>3</sup> | |||
| | |||
|- | |||
|- | |||
| bgcolor="E0E0E0" align="center" | Temp. | |||
| bgcolor="E0E0E0" align="center" | Pressure | |||
| bgcolor="E0E0E0" align="center" | ''ρ'' of liquid | |||
| bgcolor="E0E0E0" align="center" | ''ρ'' of vapor | |||
| bgcolor="E0E0E0" align="center" | ''Δ''<sub>vap</sub>''H'' | |||
|- | |||
| colspan="5" | The table above gives properties of the [[vapor-liquid equilibrium]] of anhydrous ammonia at various temperatures. The second column is vapor pressure in k[[Pascal (unit)|Pa]]. The third column is the density of the liquid phase. The fourth column is the density of the vapor. The fifth column is the heat of vaporization needed to convert one gram of liquid to vapor. | |||
|}<br clear="left"> | |||
| width="4%" | || valign="top" width="36%" | | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: {{{bgc|white}}}; border-collapse: collapse; border-color: {{{bc|#C0C090}}};" | |||
! {{chembox header}} colspan="4" | Vapor over Aqueous Ammonia Solution<ref>Perman, ''Jour. Chem. Soc. 83 1168 (1903)</ref> | |||
|- | |||
| bgcolor="E0E0E0" align="center" | Temp. | |||
| bgcolor="E0E0E0" align="center" | %wt NH<sub>3</sub> | |||
| bgcolor="E0E0E0" align="center" | Partial Pressure<br>NH<sub>3</sub> | |||
| bgcolor="E0E0E0" align="center" | Partial Pressure<br>H<sub>2</sub>O | |||
|- | |||
| rowspan="5" | 0 °C || 4.72 || 1.52 k[[Pascal (unit)|Pa]] | |||
| 0.68 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 9.15 || 3.31 k[[Pascal (unit)|Pa]] || 0.71 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 14.73 || 6.84 k[[Pascal (unit)|Pa]] || 0.55 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 19.62 || 11.0 k[[Pascal (unit)|Pa]] || 0.40 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 22.90 || 14.9 k[[Pascal (unit)|Pa]] || 0.37 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="6" | 10 °C || 4.16 || 2.20 k[[Pascal (unit)|Pa]] | |||
| 1.21 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 8.26 || 4.96 k[[Pascal (unit)|Pa]] || 1.17 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 12.32 || 8.56 k[[Pascal (unit)|Pa]] || 1.01 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 15.88 || 12.68 k[[Pascal (unit)|Pa]] | |||
| 0.93 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 20.54 || 19.89 k[[Pascal (unit)|Pa]] | |||
| 0.83 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 21.83 || 22.64 k[[Pascal (unit)|Pa]] | |||
| 0.73 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="9" | 19.9 °C || 4.18 || 3.65 k[[Pascal (unit)|Pa]] | |||
| 2.19 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 6.50 || 6.11 k[[Pascal (unit)|Pa]] || 2.15 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 6.55 || 6.13 k[[Pascal (unit)|Pa]] || 2.13 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 7.72 || 7.49 k[[Pascal (unit)|Pa]] || 2.08 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 10.15 || 10.75 k[[Pascal (unit)|Pa]] | |||
| 2.01 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 10.75 || 11.51 k[[Pascal (unit)|Pa]] | |||
| 1.96 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 16.64 || 22.14 k[[Pascal (unit)|Pa]] | |||
| 1.72 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 19.40 || 28.74 k[[Pascal (unit)|Pa]] | |||
| 1.64 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 23.37 || 40.32 k[[Pascal (unit)|Pa]] | |||
| 1.37 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="7" | 30.09 °C || 3.93 || 5.49 k[[Pascal (unit)|Pa]] | |||
| 4.15 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 7.43 || 11.51 k[[Pascal (unit)|Pa]] || 3.89 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 9.75 || 16.00 k[[Pascal (unit)|Pa]] || 3.80 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 12.77 || 23.33 k[[Pascal (unit)|Pa]] | |||
| 3.55 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 17.76 || 38.69 k[[Pascal (unit)|Pa]] | |||
| 3.31 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 17.84 || 38.81 k[[Pascal (unit)|Pa]] | |||
| 3.24 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 21.47 || 53.94 k[[Pascal (unit)|Pa]] | |||
| 2.95 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="6" | 40 °C || 3.79 || 8.15 k[[Pascal (unit)|Pa]] | |||
| 7.13 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 7.36 || 17.73 k[[Pascal (unit)|Pa]] || 6.76 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 11.06 || 29.13 k[[Pascal (unit)|Pa]] | |||
| 6.55 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 15.55 || 47.14 k[[Pascal (unit)|Pa]] | |||
| 5.52 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 17.33 || 57.02 k[[Pascal (unit)|Pa]] | |||
| | |||
|- | |||
| 20.85 || 76.81 k[[Pascal (unit)|Pa]] | |||
| 5.04 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="6" | 50 °C || 3.29 || 10.54 k[[Pascal (unit)|Pa]] | |||
| 11.95 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 5.90 || 20.17 k[[Pascal (unit)|Pa]] || 11.61 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 8.91 || 32.88 k[[Pascal (unit)|Pa]] || 11.07 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 11.57 || 45.56 k[[Pascal (unit)|Pa]] | |||
| 10.75 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 14.15 || 60.18 k[[Pascal (unit)|Pa]] | |||
| 10.27 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 14.94 || 64.94 k[[Pascal (unit)|Pa]] | |||
| 10.03 k[[Pascal (unit)|Pa]] | |||
|- | |||
| rowspan="5" | 60 °C || 3.86 || 18.25 k[[Pascal (unit)|Pa]] | |||
| 19.21 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 5.77 || 28.78 k[[Pascal (unit)|Pa]] || | |||
|- | |||
| 7.78 || 40.05 k[[Pascal (unit)|Pa]] || 18.47 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 9.37 || 50.09 k[[Pascal (unit)|Pa]] || 18.07 k[[Pascal (unit)|Pa]] | |||
|- | |||
| 9.37 || 63.43 k[[Pascal (unit)|Pa]] || 17.39 k[[Pascal (unit)|Pa]] | |||
|- | |||
| bgcolor="E0E0E0" align="center" | Temp. | |||
| bgcolor="E0E0E0" align="center" | %wt NH<sub>3</sub> | |||
| bgcolor="E0E0E0" align="center" | Partial Pressure<br>NH<sub>3</sub> | |||
| bgcolor="E0E0E0" align="center" | Partial Pressure<br>H<sub>2</sub>O | |||
|- | |||
|}<br clear="left"> | |||
|} | |||
:<math>\ | ==Heat capacity of liquid and vapor== | ||
{| | |||
|- valign="top" | |||
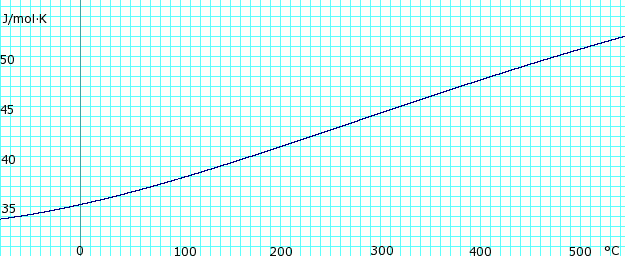
| [[Image:AmmoniaGasHeatCapacity.png|thumb|625px|left|'''Heat capacity, ''c<sub>p</sub>'', of anhydrous ammonia gas.''' Uses polynomial <!-- <math>\scriptstyle c_p (kJ/mol K) = </math><math>\scriptstyle 34.23600 - 0.02214127(T+273.15) + 1.212511 \times 10^{-4}(T+273.15)^2 - 1.088291 \times 10^{07}(T+273.15)^3 + 3.203149 \times 10^{-11}(T+273.15</math> --> obtained from CHERIC.<ref name="cheric_p">{{Cite web|url=http://www.cheric.org/research/kdb/hcprop/cmpsrch.php|title=Pure Components Properties|publisher=Chemical Engineering Research Information Center|accessdate=1 June 2007|format=Queriable database| archiveurl= http://web.archive.org/web/20070603180431/http://www.cheric.org/research/kdb/hcprop/cmpsrch.php| archivedate= 3 June 2007 <!--DASHBot-->| deadurl= no}}</ref>]] | |||
|- | |||
| [[Image:AmmoniaLiquidHeatCapacity.png|thumb|300px|left|'''Heat capacity of anhydrous liquid ammonia.''' Uses polynomial obtained from CHERIC.<ref name="cheric_p"/>]] | |||
|- | |||
|} | |||
== Spectral data == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | [[UV/VIS spectroscopy|UV-Vis]] | |||
|- | |||
| [[Lambda-max|λ<sub>max</sub>]] | |||
| None [[Nanometre|nm]] | |||
|- | |||
| [[molar absorptivity|Extinction coefficient]], ε | |||
| None | |||
|- | |||
! {{chembox header}} | [[Infrared|IR]] | |||
: | |||
: | |||
: | |||
|- | |- | ||
| | | Major absorption bands | ||
| 3444, 3337, 1627, 950 [[Wavenumber|cm]]<sup>−1</sup> | |||
|- | |- | ||
! {{chembox header}} | [[NMR Spectroscopy|NMR]] | |||
|- | |- | ||
| [[Proton NMR]] <!-- Link to image of spectrum --> | |||
| | |||
|- | |- | ||
| | | [[Carbon-13 NMR]] <!-- Link to image of spectrum --> | ||
| None - no carbons | |||
|- | |- | ||
| | | Other NMR data <!-- Insert special data e.g. <sup>19</sup>F chem. shifts, omit if not used --> | ||
| | |||
|- | |- | ||
| | ! {{chembox header}} | [[Mass Spectrometry|MS]] | ||
|- | |- | ||
| | | Masses of <br>main fragments | ||
| 17 (100%)<br/>16(80%)<br/>15(9%) | |||
|- | |- | ||
| | |} | ||
==Regulatory data== | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Regulatory data | |||
|- | |- | ||
| | | [[EINECS number]] | ||
| 231-635-3 (''gas'')<br/>215-647-6 (''soln.'') | |||
|- | |- | ||
| | | EU index number | ||
| 007-001-00-5 (''gas'')<br/>007-001-01-2 (''soln.'') | |||
|- | |- | ||
| | | [[Permissible Exposure Limit|PEL-TWA]] ([[Occupational Safety and Health Administration|OSHA]]) | ||
| 50 ppm (35 mg/m<sup>3</sup>) | |||
|- | |- | ||
| | | [[IDLH]] ([[NIOSH]]) | ||
| 300 ppm | |||
|- | |- | ||
| | | [[Flash point]] | ||
| 11 °C | |||
|- | |- | ||
| | | [[Autoignition temperature]] | ||
| 651 °C | |||
|- | |- | ||
| | | [[Explosive limits]] | ||
| 15–28% | |||
|- | |- | ||
| | | [[RTECS]] number | ||
| BO0875000 | |||
|- | |- | ||
|} | |} | ||
== | == Material Safety Data Sheet == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | ||
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet ([[Material safety data sheet|MSDS]]) for this chemical from a reliable source and follow its directions. | |||
*[http://www2.siri.org/msds/index.php SIRI] | |||
*[http://www.sciencestuff.com/msds/C1200.html Science Stuff] (Ammonia Solution) | |||
==References== | ==References== | ||
*{{ | *{{nist}} | ||
<references/> | |||
Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. | |||
[[wikipedia:Chemical infobox|Disclaimer]] applies. | |||
| | |||
==External links== | |||
*[http://webbook.nist.gov/cgi/cbook.cgi?ID=C7664417&Units=SI&Type=IR-SPEC&Index=1#IR-SPEC IR spectrum] (from [[NIST]]) | |||
{{ | {{Use dmy dates|date=September 2010}} | ||
{{DEFAULTSORT:Ammonia (Data Page)}} | |||
[[Category:Chemical data pages]] | |||
[[ | |||
Revision as of 10:51, 14 August 2014
This page provides supplementary chemical data on ammonia.
Structure and properties
| Molecular structure | |
|---|---|
| Point group | C3v |
| Bond length | 101.7 pm (N–H) |
| Bond angle | 107.8° (H–N–H) |
| Bond strength | 435 kJ/mol (H–NH2) |
| Crystal data | |
| Crystal structure | ? |
| Properties | |
| Dipole moment | 1.46 D |
| Dielectric constant | 22 ε0 at 239 K |
| Magnetic susceptibility | diamagnetic |
| Acidity of NH4+ (pKa) | 9.25 |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 195.4 K (–77.75 °C), 6.060 kPa |
| Critical point | 405.5 K (132.3 °C), 11.300 MPa |
| Std enthalpy change of fusion, ΔfusH |
+5.653 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+28.93 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+23.35 kJ/mol at BP of –33.4 °C |
| Std entropy change of vaporization, ΔvapS |
+97.41 J/(mol·K) at BP of –33.4 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 80.80 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−45.92 kJ/mol |
| Standard molar entropy, S |
192.77 J/(mol K) |
| Heat capacity, cp | 35.06 J/(mol K) |
| Heat capacity ratio, γ at 15°C |
1.310 |
| van der Waals' constants | a = 422.5 liter2 kPa / mole2 b = 0.03707 liter / mole |
Vapor-Liquid Equilibrium Data
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15600 | 30400 | 45600 |
| T in °C | –109.1(s) | –91.9(s) | –79.2(s) | –68.4 | –45.4 | –33.6 | –18.7 | 4.7 | 25.7 | 50.1 | 78.9 | 98.3 |
Table data (above) obtained from CRC Handbook of Chemistry and Physics 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid.
50 year old Petroleum Engineer Kull from Dawson Creek, spends time with interests such as house brewing, property developers in singapore condo launch and camping. Discovers the beauty in planing a trip to places around the entire world, recently only coming back from .
Vapor pressure formula for ammonia:[1]
- log10(P) = A – B / (T – C)
where P is pressure in kPa and T is temperature in kelvins
- A = 6.67956; B = 1002.711; C = 25.215 for T = 190 K through 333 K
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Heat capacity of liquid and vapor
 |
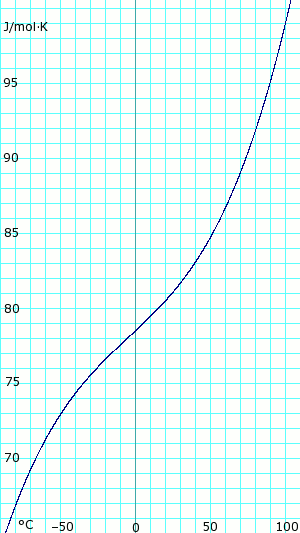 |
Spectral data
| UV-Vis | |
|---|---|
| λmax | None nm |
| Extinction coefficient, ε | None |
| IR | |
| Major absorption bands | 3444, 3337, 1627, 950 cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | None - no carbons |
| Other NMR data | |
| MS | |
| Masses of main fragments |
17 (100%) 16(80%) 15(9%) |
Regulatory data
| Regulatory data | |
|---|---|
| EINECS number | 231-635-3 (gas) 215-647-6 (soln.) |
| EU index number | 007-001-00-5 (gas) 007-001-01-2 (soln.) |
| PEL-TWA (OSHA) | 50 ppm (35 mg/m3) |
| IDLH (NIOSH) | 300 ppm |
| Flash point | 11 °C |
| Autoignition temperature | 651 °C |
| Explosive limits | 15–28% |
| RTECS number | BO0875000 |
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
- SIRI
- Science Stuff (Ammonia Solution)
References
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
External links
- IR spectrum (from NIST)
30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí.