Modular form: Difference between revisions
en>AnomieBOT m Dating maintenance tags: {{Clarify me}} |
en>Gutworth →Definition in terms of lattices or elliptic curves: \lambda is not the name of the set of lattices |
||
| Line 1: | Line 1: | ||
< | [[Image:Vap-Liq Separator.png|thumb|right|246px|A typical flash drum]] | ||
'''Flash (or partial) [[evaporation]]''' is the partial vapor that occurs when a [[Boiling point|saturated liquid]] stream undergoes a reduction in pressure by passing through a [[thermal expansion valve|throttling valve]] or other throttling device. This process is one of the simplest [[unit operation]]s. If the throttling valve or device is located at the entry into a [[pressure vessel]] so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a [[Vapor-liquid separator|flash drum]].<ref>{{cite book|author=Stanley M. Walas|title=Chemical Process Equipment:Selection and Design|edition= |publisher=Butterworth- | |||
Heinemann|year=1988|id=ISBN 0-409-90131-8 }}</ref><ref>{{cite book|author=Gas Processing Suppliers Association (GPSA)|title=Engineering Data Book|edition=10th Edition, Vol. 1|publisher=Gas Processing Suppliers Association, [[Tulsa]], [[Oklahoma]]|date=1987|id=}}</ref> | |||
If the saturated liquid is a single-component liquid (for example, liquid [[propane]] or liquid [[ammonia]]), a part of the liquid immediately "flashes" into vapor. Both the vapor and the residual liquid are cooled to the [[saturation temperature]] of the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional [[vapor compression refrigeration]] systems. | |||
If the saturated liquid is a multi-component liquid (for example, a mixture of [[propane]], [[isobutane]] and normal [[butane]]), the flashed vapor is richer in the more [[relative volatility|volatile]] components than is the remaining liquid. | |||
Uncontrolled flash evaporation can result in a boiling liquid expanding vapor explosion ([[Boiling liquid expanding vapor explosion|BLEVE]]). | |||
==Flash evaporation of a single-component liquid== | |||
The flash evaporation of a single-component liquid is an [[isenthalpic]] process and is often referred to as an '''[[Adiabatic process|adiabatic flash]]'''. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized. | |||
:<math>X = \frac{H_u^L - H_d^L}{H_d^V - H_d^L}</math> | |||
:{| border="0" cellpadding="2" | |||
|- | |||
|align=right|where: | |||
| | |||
|- | |||
!align=right|<math>X</math> | |||
|align=left|= weight fraction vaporized | |||
|- | |||
!align=right|<math>H_u^L</math> | |||
|align=left|= upstream liquid enthalpy at upstream temperature and pressure, J/kg | |||
|- | |||
!align=right|<math>H_d^V</math><br> | |||
|align=left|= flashed vapor enthalpy at downstream pressure and corresponding saturation<br> temperature, J/kg | |||
|- | |||
!align=right|<math>H_d^L</math><br> | |||
|align=left|= residual liquid enthalpy at downstream pressure and corresponding saturation<br> temperature, J/kg | |||
|} | |||
If the enthalpy data required for the above equation is unavailable, then the following equation may be used. | |||
:<math>X = \frac{c_p ( T_u - T_d )}{H_v}</math> | |||
:{| border="0" cellpadding="2" | |||
|- | |||
|align=right|where: | |||
| | |||
|- | |||
!align=right|<math>X</math> | |||
|align=left|= weight fraction vaporized | |||
|- | |||
!align=right|<math>c_p</math> | |||
|align=left|= liquid [[specific heat capacity|specific heat]] at upstream temperature and pressure, J/(kg °C) | |||
|- | |||
!align=right|<math>T_u</math> | |||
|align=left|= upstream liquid temperature, °C | |||
|- | |||
!align=right|<math>T_d</math> | |||
|align=left|= liquid [[saturation temperature]] corresponding to the downstream pressure, °C | |||
|- | |||
!align=right|<math>H_v</math><br> | |||
|align=left|= liquid [[heat of vaporization]] at downstream pressure and corresponding saturation<br> temperature, J/kg | |||
|} | |||
Here, the words "upstream" and "downstream" refer to before and after the liquid passes through the throttling valve or device. | |||
This type of flash evaporation is used in the [[desalination]] of [[brackish]] water or ocean water by "[[Multi-Stage Flash]] Distillation." The water is heated and then routed into a reduced-pressure flash evaporation "stage" where some of the water flashes into steam. This steam is subsequently condensed into salt-free water. The residual salty liquid from that first stage is introduced into a second flash evaporation stage at a pressure lower than the first stage pressure. More water is flashed into steam which is also subsequently condensed into more salt-free water. This sequential use of multiple flash evaporation stages is continued until the design objectives of the system are met. A large part of the world's installed desalination capacity uses multi-stage flash distillation. Typically such plants have 24 or more sequential stages of flash evaporation. | |||
==Equilibrium flash of a multi-component liquid== | |||
The '''equilibrium flash''' of a multi-component liquid may be visualized as a simple [[distillation]] process using a single [[equilibrium stage]]. It is very different and more complex than the flash evaporation of single-component liquid. For a multi-component liquid, calculating the amounts of flashed vapor and residual liquid in equilibrium with each other at a given temperature and pressure requires a trial-and-error [[Iterative method|iterative]] solution. Such a calculation is commonly referred to as an equilibrium flash calculation. It involves solving the ''Rachford-Rice equation'':<ref> | |||
{{cite book|author=Harry Kooijman and Ross Taylor|url=http://www.chemsep.com/downloads/docs/book2.pdf|title=The ChemSep Book|edition=2nd |publisher=|year=2000|id=ISBN 3-8311-1068-9}} See page 186.</ref><ref>[https://www.e-education.psu.edu/png520/m13_p2.html Analysis of Objective Functions] (Pennsylvania State University)</ref><ref>[http://www.mathworks.com/matlabcentral/fileexchange/loadFile.do?objectId=9341&objectType=file Flash Calculations using the Soave-Redlich-Kwong equation of state] (view full-size image)</ref><ref name="whitson">Curtis H. Whitson, Michael L. Michelsen, ''The Negative Flash'', Fluid Phase Equilibria, 53 (1989) 51–71.</ref> | |||
:<math>\sum_i\frac{z_i \, (K_i - 1)}{1 + \beta \, (K_i - 1)}=0</math> | |||
where: | |||
* ''z<sub>i</sub>'' is the mole fraction of component ''i'' in the feed liquid (assumed to be known); | |||
* ''β'' is the fraction of feed that is vaporised; | |||
* ''K<sub>i</sub>'' is the equilibrium constant of component ''i''. | |||
The equilibrium constants ''K<sub>i</sub>'' are in general functions of many parameters, though the most important is arguably temperature; they are defined as: | |||
:<math>y_i = K_i \, x_i</math> | |||
where: | |||
* ''x<sub>i</sub>'' is the mole fraction of component ''i'' in liquid phase; | |||
* ''y<sub>i</sub>'' is the mole fraction of component ''i'' in gas phase. | |||
Once the Rachford-Rice equation has been solved for ''β'', the compositions ''x<sub>i</sub>'' and ''y<sub>i</sub>'' can be immediately calculated as: | |||
:<math>\begin{align} | |||
x_i &= \frac{z_i}{1+\beta(K_i-1)}\\ | |||
y_i &= K_i\,x_i. | |||
\end{align}</math> | |||
The Rachford-Rice equation can have multiple solutions for ''β'', at most one of which guarantees that all ''x<sub>i</sub>'' and ''y<sub>i</sub>'' will be positive. In particular, if there is only one ''β'' for which: | |||
:<math>\frac{1}{1-K_\text{max}} = \beta_\text{min} < \beta < \beta_\text{max} = \frac{1}{1-K_\text{min}}</math> | |||
then that ''β'' is the solution; if there are multiple such ''β'''s, it means that either ''K''<sub>max</sub><1 or ''K''<sub>min</sub>>1, indicating respectively that no gas phase can be sustained (and therefore ''β''=0) or conversely that no liquid phase can exist (and therefore ''β''=1). | |||
It is possible to use [[Newton's method]] for solving the above water equation, but there is a risk of converging to the wrong value of ''β''; it is important to initialise the solver to a sensible initial value, such as (''β<sub>max</sub>''+''β<sub>min</sub>'')/2 (which is however not sufficient: Newton's method makes no guarantees on stability), or, alternatively, use a bracketing solver such as the [[bisection method]] or the [[Brent method]], which are guaranteed to converge but can be slower. | |||
The equilibrium flash of multi-component liquids is very widely utilized in [[Oil refinery|petroleum refineries]], [[petrochemical]] and [[chemical plant]]s and [[natural gas processing]] plants. | |||
==Contrast with spray drying== | |||
[[Spray drying]] is sometimes seen as a form of flash evaporation. However, although it is a form of liquid evaporation, it is quite different from flash evaporation. | |||
In spray drying, a [[slurry]] of very small solids is rapidly dried by suspension in a hot gas. The slurry is first [[Aerosol|atomized]] into very small liquid droplets which are then sprayed into a stream of hot dry air. The liquid rapidly evaporates leaving behind dry powder or dry solid granules. The dry powder or solid granules are recovered from the exhaust air by using [[cyclone (industry)|cyclones]], [[Filtration|bag filters]] or [[electrostatic precipitator]]s. | |||
==Natural flash evaporation== | |||
Natural flash vaporization or flash deposition may occur during [[earthquake]]s resulting in depositing of minerals held in [[Supersaturation|supersaturated solution]]s, sometimes even valuable [[ore]] in the case of auriferous, gold-bearing, waters. This results when blocks of rock are rapidly pulled and pushed away from each other by [[Fault (geology)|jog fault]]s.<ref name=SA031813>{{cite news|title=Earthquakes Make Gold Veins in an Instant: Pressure changes cause the precious metal to deposit each time the crust moves, a new study finds. The insight suggests that remote sensing could be used to find new deposits in rocks where fault jogs are common|url=http://www.scientificamerican.com/article.cfm?id=earthquakes-make-gold-veins-in-an-instant|accessdate=March 18, 2013|newspaper=Scientific American|date=March 18, 2013|author=Richard A. Lovett|author2=Nature magazine}}</ref> | |||
==See also== | |||
*[[Evaporator]] | |||
*[[Vapor-liquid separator]] | |||
==References== | |||
{{reflist}} | |||
==External links== | |||
*[http://www.tlv.com/global/US/steam-theory/flash-steam-and-vapor.html Vapor and Flash Steam] Animation, photos and technical explanation of the difference between Flash Steam and Vaporized fraction. | |||
*[http://www.spiraxsarco.com/resources/steam-engineering-tutorials/condensate-recovery/flash-steam.asp Flash Steam Tutorial] The benefits of recovering flash steam, how it is done and typical applications. | |||
*[http://www.escwa.org.lb/information/publications/edit/upload/tech-01-3-e.pdf Water Desalination Technologies] in the Middle East and Western Asia | |||
*[http://www.niro.com/ndk_website/niro/cmsresources.nsf/filenames/spray-drying.pdf/$file/spray-drying.pdf Discussion of spray drying] | |||
*[http://petrochemical.gronerth.com/flash_distillation_program_explorer.htm Flash evaporation program online] Flash distillation of the hydrocarbon compounds. | |||
{{States of matter}} | |||
[[Category:Evaporators]] | |||
[[Category:Chemical processes]] | |||
[[Category:Fluid dynamics]] | |||
[[Category:Heating, ventilating, and air conditioning]] | |||
[[Category:Thermodynamics]] | |||
[[Category:Unit operations]] | |||
Revision as of 00:19, 22 January 2014
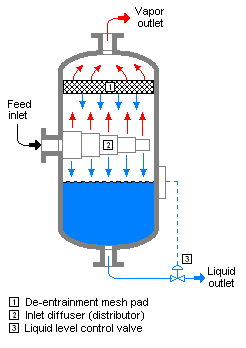
Flash (or partial) evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations. If the throttling valve or device is located at the entry into a pressure vessel so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a flash drum.[1][2]
If the saturated liquid is a single-component liquid (for example, liquid propane or liquid ammonia), a part of the liquid immediately "flashes" into vapor. Both the vapor and the residual liquid are cooled to the saturation temperature of the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional vapor compression refrigeration systems.
If the saturated liquid is a multi-component liquid (for example, a mixture of propane, isobutane and normal butane), the flashed vapor is richer in the more volatile components than is the remaining liquid.
Uncontrolled flash evaporation can result in a boiling liquid expanding vapor explosion (BLEVE).
Flash evaporation of a single-component liquid
The flash evaporation of a single-component liquid is an isenthalpic process and is often referred to as an adiabatic flash. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized.
If the enthalpy data required for the above equation is unavailable, then the following equation may be used.
where: = weight fraction vaporized Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle c_{p}} = liquid specific heat at upstream temperature and pressure, J/(kg °C) = upstream liquid temperature, °C = liquid saturation temperature corresponding to the downstream pressure, °C
= liquid heat of vaporization at downstream pressure and corresponding saturation
temperature, J/kg
Here, the words "upstream" and "downstream" refer to before and after the liquid passes through the throttling valve or device.
This type of flash evaporation is used in the desalination of brackish water or ocean water by "Multi-Stage Flash Distillation." The water is heated and then routed into a reduced-pressure flash evaporation "stage" where some of the water flashes into steam. This steam is subsequently condensed into salt-free water. The residual salty liquid from that first stage is introduced into a second flash evaporation stage at a pressure lower than the first stage pressure. More water is flashed into steam which is also subsequently condensed into more salt-free water. This sequential use of multiple flash evaporation stages is continued until the design objectives of the system are met. A large part of the world's installed desalination capacity uses multi-stage flash distillation. Typically such plants have 24 or more sequential stages of flash evaporation.
Equilibrium flash of a multi-component liquid
The equilibrium flash of a multi-component liquid may be visualized as a simple distillation process using a single equilibrium stage. It is very different and more complex than the flash evaporation of single-component liquid. For a multi-component liquid, calculating the amounts of flashed vapor and residual liquid in equilibrium with each other at a given temperature and pressure requires a trial-and-error iterative solution. Such a calculation is commonly referred to as an equilibrium flash calculation. It involves solving the Rachford-Rice equation:[3][4][5][6]
where:
- zi is the mole fraction of component i in the feed liquid (assumed to be known);
- β is the fraction of feed that is vaporised;
- Ki is the equilibrium constant of component i.
The equilibrium constants Ki are in general functions of many parameters, though the most important is arguably temperature; they are defined as:
where:
- xi is the mole fraction of component i in liquid phase;
- yi is the mole fraction of component i in gas phase.
Once the Rachford-Rice equation has been solved for β, the compositions xi and yi can be immediately calculated as:
The Rachford-Rice equation can have multiple solutions for β, at most one of which guarantees that all xi and yi will be positive. In particular, if there is only one β for which:
then that β is the solution; if there are multiple such β's, it means that either Kmax<1 or Kmin>1, indicating respectively that no gas phase can be sustained (and therefore β=0) or conversely that no liquid phase can exist (and therefore β=1).
It is possible to use Newton's method for solving the above water equation, but there is a risk of converging to the wrong value of β; it is important to initialise the solver to a sensible initial value, such as (βmax+βmin)/2 (which is however not sufficient: Newton's method makes no guarantees on stability), or, alternatively, use a bracketing solver such as the bisection method or the Brent method, which are guaranteed to converge but can be slower.
The equilibrium flash of multi-component liquids is very widely utilized in petroleum refineries, petrochemical and chemical plants and natural gas processing plants.
Contrast with spray drying
Spray drying is sometimes seen as a form of flash evaporation. However, although it is a form of liquid evaporation, it is quite different from flash evaporation.
In spray drying, a slurry of very small solids is rapidly dried by suspension in a hot gas. The slurry is first atomized into very small liquid droplets which are then sprayed into a stream of hot dry air. The liquid rapidly evaporates leaving behind dry powder or dry solid granules. The dry powder or solid granules are recovered from the exhaust air by using cyclones, bag filters or electrostatic precipitators.
Natural flash evaporation
Natural flash vaporization or flash deposition may occur during earthquakes resulting in depositing of minerals held in supersaturated solutions, sometimes even valuable ore in the case of auriferous, gold-bearing, waters. This results when blocks of rock are rapidly pulled and pushed away from each other by jog faults.[7]
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- Vapor and Flash Steam Animation, photos and technical explanation of the difference between Flash Steam and Vaporized fraction.
- Flash Steam Tutorial The benefits of recovering flash steam, how it is done and typical applications.
- Water Desalination Technologies in the Middle East and Western Asia
- Discussion of spray drying
- Flash evaporation program online Flash distillation of the hydrocarbon compounds.
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 See page 186. - ↑ Analysis of Objective Functions (Pennsylvania State University)
- ↑ Flash Calculations using the Soave-Redlich-Kwong equation of state (view full-size image)
- ↑ Curtis H. Whitson, Michael L. Michelsen, The Negative Flash, Fluid Phase Equilibria, 53 (1989) 51–71.
- ↑ Template:Cite news












