Projective plane


A peptide bond (amide bond) is a covalent chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, causing the release of a molecule of water (H2O), hence the process is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids. The resulting C(O)NH bond is called a peptide bond, and the resulting molecule is an amide. The four-atom functional group -C(=O)NH- is called a peptide link. Polypeptides and proteins are chains of amino acids held together by peptide bonds, as is the backbone of PNA.
A peptide bond can be broken by hydrolysis (the adding of water). In the presence of water they will break down and release 8–16 kilojoule/mol (2–4 kcal/mol) [1] of free energy. This process is extremely slow (up to 1000 years). In living organisms, the process is facilitated by enzymes. Living organisms also employ enzymes to form peptide bonds; this process requires free energy. The wavelength of absorbance for a peptide bond is 190–230 nm[2] (which makes it particularly susceptible to UV radiation).

Cis/trans isomers of the peptide group
Significant delocalisation of the lone pair of electrons on the nitrogen atom gives the group a partial double bond character. The partial double bond renders the amide group planar, occurring in either the cis or trans isomers. In the unfolded state of proteins, the peptide groups are free to isomerize and adopt both isomers; however, in the folded state, only a single isomer is adopted at each position (with rare exceptions). The trans form is preferred overwhelmingly in most peptide bonds (roughly 1000:1 ratio in trans:cis populations). However, X-Pro peptide groups tend to have a roughly 3:1 ratio, presumably because the symmetry between the and atoms of proline makes the cis and trans isomers nearly equal in energy (See figure, below).
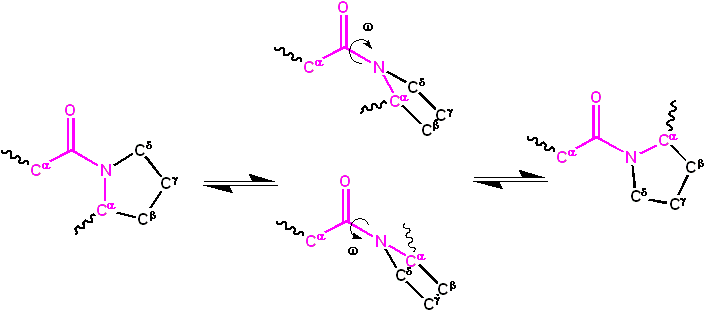
The dihedral angle associated with the peptide group (defined by the four atoms ) is denoted ; for the cis isomer and for the trans isomer. Amide groups can isomerize about the C-N bond between the cis and trans forms, albeit slowly (20 seconds at room temperature). The transition states requires that the partial double bond be broken, so that the activation energy is roughly 80 kilojoule/mol (20 kcal/mol) (See Figure below). However, the activation energy can be lowered (and the isomerization catalyzed) by changes that favor the single-bonded form, such as placing the peptide group in a hydrophobic environment or donating a hydrogen bond to the nitrogen atom of an X-Pro peptide group. Both of these mechanisms for lowering the activation energy have been observed in peptidyl prolyl isomerases (PPIases), which are naturally occurring enzymes that catalyze the cis-trans isomerization of X-Pro peptide bonds.
Conformational protein folding is usually much faster (typically 10–100 ms) than cis-trans isomerization (10–100 s). A nonnative isomer of some peptide groups can disrupt the conformational folding significantly, either slowing it or preventing it from even occurring until the native isomer is reached. However, not all peptide groups have the same effect on folding; nonnative isomers of other peptide groups may not affect folding at all.
Chemical reactions
Due to its resonance stabilization, the peptide bond is relatively unreactive under physiological conditions, even less than similar compounds such as esters. Nevertheless, peptide bonds can undergo chemical reactions, usually through an attack of an electronegative atom on the carbonyl carbon, breaking the carbonyl double bond and forming a tetrahedral intermediate. This is the pathway followed in proteolysis and, more generally, in N-O acyl exchange reactions such as those of inteins. When the functional group attacking the peptide bond is a thiol, hydroxyl or amine, the resulting molecule may be called a cyclol or, more specifically, a thiacyclol, an oxacyclol or an azacyclol, respectively.
See also
References
- ↑ Martin RB. (1998) "Free energies and equilibria of peptide bond hydrolysis and formation", Biopolymers, 45, 351–353.
- ↑ Goldfarb AR et al. (1951) "The Ultraviolet Absorption Spectra of Proteins", J. Biological Chem., 193, 397–404.(http://www.jbc.org/content/193/1/397.long)
- Pauling L. (1960) The Nature of the Chemical Bond, 3rd. ed., Cornell University Press. ISBN 0-8014-0333-2
- Stein RL. (1993) "Mechanism of Enzymatic and Nonenzymatic Prolyl cis-trans Isomerization", Adv. Protein Chem., 44, 1–24.
- Schmid FX, Mayr LM, Mücke M and Schönbrunner ER. (1993) "Prolyl Isomerases: Role in Protein Folding", Adv. Protein Chem., 44, 25–66.
- Fischer G. (1994) "Peptidyl-Prolyl cis/trans Isomerases and Their Effectors", Angew. Chem. Int. Ed. Engl., 33, 1415–1436.







