Rodrigues' rotation formula

In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds (refer to figure on single bond rotation).[1] Such isomers are generally referred to as conformational isomers or conformers and, specifically, as rotamers.[2] Rotations about single bonds are restricted by a rotational energy barrier which must be overcome to interconvert one conformer to another. Conformational isomerism arises when the rotation about a single bond is relatively unhindered. That is, the energy barrier must be small enough for the interconversion to occur.
Conformational isomers are thus distinct from the other classes of stereoisomers (i. e. configurational isomers) where interconversion necessarily involves breaking and reforming of chemical bonds.[3] For example, L- & D and R- & S- configurations of organic molecules have different handedness and optical activities, and can only be interconverted by breaking one or more bonds connected to the chiral atom and reforming a similar bond in a different direction or spatial orientation.
The study of the energetics between different rotamers is referred to as conformational analysis.[4] It is useful for understanding the stability of different isomers, for example, by taking into account the spatial orientation and through-space interactions of substituents. In addition, conformational analysis can be used to predict and explain product(s) selectivity, mechanisms, and rates of reactions.[5]
Types of conformational isomerism

The types of conformational isomers are related to the spatial orientations of the substituents between two vicinal atoms. These are eclipsed and staggered. The staggered conformation includes the gauche (±60°) and anti (180°) conformations, depending on the spatial orientations of the two substituents.
For example, butane has three rotamers relating to its two methyl (CH3) groups: two gauche conformers, which have the methyls ±60° apart and are enantiomeric, and an anti conformer, where the four carbon centres are coplanar and the substituents are 180° apart (refer to free energy diagram of butane). The energy difference between gauche and anti is 0.9 kcal/mol associated with the strain energy of the gauche conformer.[4] The anti conformer is, therefore, the most stable (~0 kcal/mol). The three eclipsed conformations with dihedral angles of 0°,120° and 240° are not considered to be rotamers, but are instead transition states of higher energy.[4] Note that the two eclipsed conformations have different energies: at 0° the two methyl groups are eclipsed, resulting in higher energy (~5 kcal/mol) than at 120°, where the methyl groups are eclipsed with hydrogens (~3.5 kcal/mol).[6]
While simple molecules can be described by these types of conformations, more complex molecules require the use of the Klyne-Prelog system to describe the different conformers.[4]
More specific examples of conformational isomerism are detailed elsewhere:
- Ring conformation
- Cyclohexane conformations with chair and boat conformers.
- Carbohydrate conformation.
- Allylic strain - energetics related to rotation about the single bond between sp2 and sp3 carbons.
- Atropisomerism- due to restricted rotation about a bond, a molecule can become chiral.
- Folding of molecules, where some shapes are stable and functional, but others are not.
Free energy and equilibria of conformational isomers
Equilibrium of conformers

Conformational isomers exist in a dynamic equilibrium, where the relative free energies of isomers determines the population of each isomer and the energy barrier of rotation determines the rate of interconversion between isomers:[7]
where is the equilibrium constant, is the change in free energy for the interconversion of two conformers in kcal/mol, is the universal gas constant (0.002 kcal/mol K), and is the system's temperature in Kelvin (K).
Three isotherms are given in the diagram depicting the equilibrium distribution of two conformers at different temperatures. Note that a 0 kcal/mol free energy change gives an equilibrium constant of 1, meaning that two conformers have equal stability and exist in a 1:1 ratio. A negative change in free energy means that a conformer interconverts to a thermodynamically favored conformation, thus the equilibrium constant will always be greater than 1. For example, the ΔG of butane from gauche to anti is -0.9 kcal/mol, therefore the equilibrium constant is 4.5, favoring the anti conformation. Also notice that at large positive ΔG (i.e. unlikely for interconversion to occur), the equilibrium constant between two conformers can be increased by increasing temperature.
Population distribution of conformers
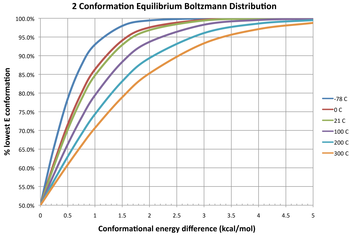
The fractional population distribution of different conformers follows a Boltzmann distribution:[8]
The left hand side is the equilibrium ratio of conformer i to the total. is the relative energy of the i-th conformer from the minimum energy conformer. is the relative energy of the k-th conformer from the minimum energy conformer. R is the molar ideal gas constant equal to 8.31 J/(mol·K) and T is the temperature in kelvins (K). The denominator of the right side is the partition function.
Factors contributing to the free energy of conformers
The effects of electrostatic and steric interactions of the substituents as well as orbital interactions such as hyperconjugation are responsible for the relative stability of conformers and their transition states. The contributions of these factors vary depending on the nature of the substituents and may either contribute positively or negatively to the energy barrier. Computational studies of small molecules such as ethane suggest that electrostatic effects make the greatest contribution to the energy barrier; however, the barrier is traditionally attributed primarily to steric interactions.[9][10]
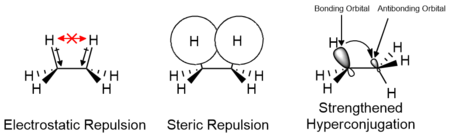
In the case of cyclic systems, the steric effect and contribution to the free energy can be approximated by A values, which measure the energy difference when a substituent is in the axial or equatorial position.
Isolation or observation of the conformational isomers
The short timescale of interconversion precludes the separation of conformational isomers in many cases. Atropisomers are conformational isomers which can be separated due to restricted rotation.[11]
Protein folding also generates stable conformational isomers which can be observed. The Karplus equation relates the dihedral angle of vicinal protons to their J-coupling constants as measured by NMR. The equation aids in the elucidation of protein folding as well as the conformations of other rigid aliphatic molecules.[12]
The equilibrium between conformational isomers may also be observed using spectroscopic techniques:
In cyclohexane derivatives, the two chair conformers interconvert with rates on the order of 105ring flips/sec, which precludes their separation.[13] The conformer in which the substituent is equitorial crystallizes selectively, and when these crystals are dissolved at very low temperatures, one can directly monitor the approach to equilibrium by NMR spectroscopy.[14]
The dynamics of conformational (and other kinds of) isomerism can be monitored by NMR spectroscopy at varying temperatures. The technique applies to barriers of 8–14 kcal/mol, and species exhibiting such dynamics are often called "fluxional".
IR spectroscopy is ordinarily used to measure conformer ratios. For the axial and equatorial conformer of bromocyclohexane, νCBr differs by almost 50 cm−1.[13]
Conformation-dependent reactions
Reaction rates are highly dependent on the conformation of the reactants. This theme is especially well elucidated in organic chemistry. One example is provided by the elimination reactions, which involve the simultaneous removal of a proton and a leaving group from vicinal positions under the influence of a base.
The mechanism requires that the departing atoms or groups follow antiparallel trajectories. For open chain substrates this geometric prerequisite is met by at least one of the three staggered conformers. For some cyclic substrates such as cyclohexane, however, an antiparallel arrangement may not be attainable depending on the substituents which might set a conformational lock.[15] Adjacent substituents on a cyclohexane ring can achieve antiperiplanarity only when they occupy trans diaxial positions.
One consequence of this analysis is that trans-4-tert-butylcyclohexyl chloride cannot easily eliminate but instead undergoes substitution (see diagram below) because the most stable conformation has the bulky tBu group in the equatorial position, therefore the chloride group is not antiperiplanar with any vicinal hydrogen. The thermodynamically unfavored conformation has the tBu group in the axial position, which exhibits the high energetic 7-atoms interactions (see A value) of 4.7 - 4.9 kcal/mol.[16] As a result, the tBu group "locks" the ring in the conformation where it is in the equatorial position and substitution reaction is observed. On the other hand, cis-4-tert-butylcyclohexyl chloride undergoes elimination because antiperiplanarity of Cl and H can be achieved when the tBu group is in the favorable equatorial position.
See also
- Isomer
- Steric effects
- Molecular configuration
- Macrocyclic stereocontrol
- Klyne-Prelog System
- Anomeric effect
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- ↑ IUPAC definition of a conformer.
- ↑ Template:GoldBookRef
- ↑ Template:Cite web
- ↑ 4.0 4.1 4.2 4.3 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Template:Cite web
- ↑ 13.0 13.1 Eliel, E. L.; Wilen, S. H.; Mander, L. N. "Stereochemistry Of Organic Compounds", J. Wiley and Sons, 1994. ISBN 0-471-01670-5.
- ↑ Template:Cite doi
- ↑ Template:Cite web
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534







