Bernstein polynomial: Difference between revisions
en>777sms No edit summary |
Consistently as a function of x again, and as b not B |
||
| Line 1: | Line 1: | ||
'''Electron ionization''' ('''EI''', formerly known as '''electron impact''') is an [[ionization]] method in which energetic [[electron]]s interact with gas phase atoms or molecules to produce [[ion]]s.<ref>{{GoldBookRef|title=electron ionization|file=E01999}}</ref> This technique is widely used in [[mass spectrometry]], particularly for gases and volatile [[organic chemistry|organic molecules]]. | |||
==Principle of operation== | |||
[[Image:Schematic diagram of an EI ion source.jpg|thumb|right|300 px|Diagram representing an electron ionization ion source]] | |||
The following [[Phase (matter)|gas phase]] reaction describes the electron ionization process:<ref>R. Davis, M. Frearson, (1987). ''Mass Spectrometry – Analytical Chemistry by Open Learning'', John Wiley & Sons, London.</ref> | |||
:<math>M + e^- \to M^{+\bullet} + 2e^-,</math> | |||
where M is the analyte molecule being ionized, e<sup>−</sup> is the electron and M<sup>+•</sup> is the resulting [[ion]]. | |||
In an EI [[ion source]], electrons are produced through [[thermionic emission]] by heating a wire filament that has [[electric current]] running through it. The electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The accelerated electrons are then concentrated into a beam by being attracted to the trap electrode. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons, referred to as a ''hard'' ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation.<ref>K. Robinson ''et al.'' Undergraduate Instrumental Analysis, 6th ed. Marcel Drekker, New York, 2005.</ref> The [[radical ion|radical cation]] products are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte. | |||
The [[ionization efficiency]] and production of fragment ions depends strongly on the chemistry of the analyte and the energy of the electrons. At low energies (around 20 [[Electronvolt|eV]]), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the [[Matter wave|de Broglie wavelength]] of the electrons matches the length of typical bonds in organic molecules (about 0.14 [[Nanometre|nm]]) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytes; the molecules then become "transparent" to the electrons and ionization efficiency decreases. | |||
==Notes== | |||
{{Reflist}} | |||
==References== | |||
{{refbegin}} | |||
*{{cite book | author = Edmond de Hoffman | coauthors = Vincent Stroobant | title = Mass Spectrometry: Principles and Applications | edition = 2nd ed. | publisher = John Wiley and Sons | year = 2001 | isbn = 0-471-48566-7}} | |||
*{{cite book |author=Stephen J. Schrader |title=Interpretation of Electron Ionization Data: The Odd Book |publisher=Not Avail |location= |year=2001 |pages= |isbn=0-9660813-6-6 |oclc= |doi= |accessdate=}} | |||
*{{cite book |author=Peterkops, Raimonds |title=Theory of ionization of atoms by electron impact |publisher=Colorado Associated University Press |location=Boulder, Colo |year=1977 |pages= |isbn=0-87081-105-3 |oclc= |doi= |accessdate=}} | |||
*{{cite book |author= |title=Electron impact ionization |publisher=Springer-Verlag |location=Berlin |year=1985 |pages= |isbn=0-387-81778-6 |oclc= |doi= |accessdate=}} | |||
{{refend}} | |||
==External links== | |||
* [http://webbook.nist.gov/cgi/cbook.cgi?ID=C108883&Units=SI&Mask=200#Mass-Spec NIST Chemistry WebBook] | |||
* [http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/Spectrpy/MassSpec/masspec1.htm Mass Spectrometry]. Michigan State University. | |||
{{Mass spectrometry}} | |||
{{DEFAULTSORT:Electron Ionization}} | |||
[[Category:Ion source]] | |||
[[Category:Mass spectrometry]] | |||
[[Category:Scientific techniques]] | |||
Revision as of 15:57, 5 January 2014
Electron ionization (EI, formerly known as electron impact) is an ionization method in which energetic electrons interact with gas phase atoms or molecules to produce ions.[1] This technique is widely used in mass spectrometry, particularly for gases and volatile organic molecules.
Principle of operation
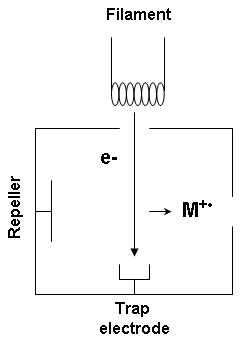
The following gas phase reaction describes the electron ionization process:[2]
where M is the analyte molecule being ionized, e− is the electron and M+• is the resulting ion.
In an EI ion source, electrons are produced through thermionic emission by heating a wire filament that has electric current running through it. The electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The accelerated electrons are then concentrated into a beam by being attracted to the trap electrode. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons, referred to as a hard ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation.[3] The radical cation products are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte.
The ionization efficiency and production of fragment ions depends strongly on the chemistry of the analyte and the energy of the electrons. At low energies (around 20 eV), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the de Broglie wavelength of the electrons matches the length of typical bonds in organic molecules (about 0.14 nm) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytes; the molecules then become "transparent" to the electrons and ionization efficiency decreases.
Notes
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
References
- 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534
External links
- NIST Chemistry WebBook
- Mass Spectrometry. Michigan State University.
- ↑ Template:GoldBookRef
- ↑ R. Davis, M. Frearson, (1987). Mass Spectrometry – Analytical Chemistry by Open Learning, John Wiley & Sons, London.
- ↑ K. Robinson et al. Undergraduate Instrumental Analysis, 6th ed. Marcel Drekker, New York, 2005.
