Proof of Bertrand's postulate: Difference between revisions
en>Touchatou m Remove redirection |
en>Addbot m Bot: Migrating 1 interwiki links, now provided by Wikidata on d:q13634063 |
||
| Line 1: | Line 1: | ||
[[File:Galvanic cell with no cation flow.png|400px|right]] | |||
A '''galvanic cell''', or '''voltaic cell''', named after [[Luigi Galvani]], or [[Alessandro Volta]] respectively, is an [[electrochemical cell]] that derives electrical energy from spontaneous [[redox]] reactions taking place within the cell. It generally consists of two different metals connected by a [[salt bridge]], or individual half-cells separated by a porous membrane. | |||
Volta was the inventor of the [[voltaic pile]], the first [[Battery (electricity)|electrical battery]]. In common usage, the word "battery" has come to include a single galvanic cell, but a battery properly consists of multiple cells.<ref>[http://www.merriam-webster.com/dictionary/battery "battery" (def. 4b)], ''Merriam-Webster Online Dictionary'' (2008). Retrieved 6 August 2008.</ref> | |||
==History== | |||
In 1780, [[Luigi Galvani]] discovered that when two different [[metal]]s (e.g., copper and zinc) are connected and then both touched at the same time to two different parts of a nerve of a frog leg, then the leg contracts.<ref>{{cite book | |||
| title = Daniell Cell | |||
| last = Keithley | first = Joseph F. | |||
| publisher = John Wiley and Sons | |||
| year = 1999 | |||
| isbn = 0-7803-1193-0 | |||
| pages = 49–51 | |||
}}</ref> | |||
He called this "[[bioelectricity|animal electricity]]". The voltaic pile, invented by [[Alessandro Volta]] in the 1800s, consists of a pile of cells similar to the galvanic cell. However, Volta built it entirely out of non-biological material in order to challenge Galvani's (and the later experimenter [[Leopoldo Nobili]]) animal electricity theory in favour of his own metal-metal contact electricity theory.<ref>Kipnis, pages 144-146</ref> [[Carlo Matteucci]] in his turn constructed a [[Frog battery|battery entirely out of biological material]] in answer to Volta.<ref>Clarke & Jacyna, page 199</ref> These discoveries paved the way for electrical batteries; Volta's cell was named an [[List of IEEE milestones|IEEE Milestone]] in 1999.<ref>{{cite web |url=http://www.ieeeghn.org/wiki/index.php/Milestones:Volta%27s_Electrical_Battery_Invention,_1799 |title=Milestones:Volta's Electrical Battery Invention, 1799 |author= |date= |work=IEEE Global History Network |publisher=IEEE |accessdate=26 July 2011}}</ref> | |||
It was suggested by [[Wilhelm König]] in 1940 that the object known as the [[Baghdad battery]] might represent galvanic cell technology from ancient [[Parthia]]. Replicas filled with citric acid or grape juice have been shown to produce a voltage. However, it is far from certain that this was its purpose—other scholars have pointed out that it is very similar to vessels known to have been used for storing parchment scrolls.<ref>Brian Haughton, ''Hidden History: Lost Civilizations, Secret Knowledge, and Ancient Mysteries'', pages 129-132, Career Press, 2007 ISBN 1564148971.</ref> | |||
==Description== | |||
[[Image:Galvanic cell labeled.svg|350px|thumb|Schematic of Zn-Cu galvanic cell]] | |||
In its simplest form, a [[half-cell]] consists of a solid metal (called an [[electrode]]) that is submerged in a solution; the solution contains [[cation]]s of the electrode metal and [[anion]]s to balance the charge of the cations. In essence, a half-cell contains a metal in two [[oxidation state]]s; inside an isolated half-cell, there is an [[redox|oxidation-reduction]] (redox) reaction that is in [[chemical equilibrium]], a condition written symbolically as follows (here, "M" represents a metal cation, an atom that has a charge imbalance due to the loss of "''n''" electrons): | |||
: M<sup>''n''+</sup> (oxidized species) + ''n''e<sup>-</sup> {{eqm}} M (reduced species) | |||
A galvanic cell consists of two half-cells, such that the electrode of one half-cell is composed of metal A, and the electrode of the other half-cell is composed of metal B; the redox reactions for the two separate half-cells are thus: | |||
: A<sup>''n''+</sup> + ''n''e<sup>-</sup> {{eqm}} A | |||
: B<sup>''m''+</sup> + ''m''e<sup>-</sup> {{eqm}} B | |||
In general, then, these two metals can react with each other: | |||
: ''m'' A + ''n'' B<sup>''m''+</sup> {{eqm}} ''n'' B + ''m'' A<sup>''n''+</sup> | |||
In other words, the metal atoms of one half-cell are able to induce reduction of the metal cations of the other half-cell; conversely stated, the metal cations of one half-cell are able to oxidize the metal atoms of the other half-cell. When metal B has a greater [[electronegativity]] than metal A, then metal B tends to steal electrons from metal A (that is, metal B tends to oxidize metal A), thus favoring one direction of the reaction: | |||
: ''m'' A + ''n'' B<sup>''m''+</sup> <math>\rightarrow</math> ''n'' B + ''m'' A<sup>''n''+</sup> | |||
This reaction between the metals can be controlled in a way that allows for doing useful [[Work (physics)|work]]: | |||
* The electrodes are connected with a metal wire in order [[Electrical conduction|to conduct]] the electrons that participate in the reaction. | |||
: In one half-cell, dissolved metal-B cations combine with the [[free electron]]s that are available at the interface between the solution and the metal-B electrode; these cations are thereby neutralized, causing them to [[Precipitation (chemistry)|precipitate]] from solution as deposits on the metal-B electrode, a process known as [[plating]]. | |||
: This reduction reaction causes the free electrons throughout the metal-B electrode, the wire, and the metal-A electrode to be pulled into the metal-B electrode. Consequently, electrons are wrestled away from some of the atoms of the metal-A electrode, as though the metal-B cations were reacting directly with them; those metal-A atoms become cations that dissolve into the surrounding solution. | |||
: As this reaction continues, the half-cell with the metal-A electrode develops a positively charged solution (because the metal-A cations dissolve into it), while the other half-cell develops a negatively charged solution (because the metal-B cations precipitate out of it, leaving behind the anions); unabated, this imbalance in charge would stop the reaction. | |||
* The solutions are connected by a salt bridge or a porous plate in order to conduct the ions (both the metal-A cations from one solution, and the anions from the other solution), which balances the charges of the solutions and thereby allows the reaction between metal A and metal B to continue without opposition. | |||
By definition: | |||
* The [[anode]] is the electrode where oxidation (loss of electrons) takes place; the <u>an</u>ode attracts <u>an</u>ions. The metal-A electrode is the anode. | |||
* The [[cathode]] is the electrode where reduction (gain of electrons) takes place; the <u>cat</u>hode attracts <u>cat</u>ions. The metal-B electrode is the cathode. | |||
Copper readily oxidizes zinc; for the [[Daniell cell]] depicted in the figure, the anode is [[zinc]] and the cathode is [[copper]], and the anions in the solutions are [[sulfate]]s of the respective metals. When an electrically conducting device connects the electrodes, the electrochemical reaction is: | |||
: Zn + {{chem|Cu|2+}} → {{chem|Zn|2+}}+ Cu | |||
The zinc electrode is dissolved and copper is deposited on the copper electrode. | |||
Galvanic cells are typically used as a source of electrical power. By their nature, they produce [[direct current]]. The [[Weston cell]] has an anode composed of [[cadmium]] [[mercury (element)|mercury]] [[amalgam (chemistry)|amalgam]], and a cathode composed of pure mercury. The electrolyte is a (saturated) solution of [[cadmium sulfate]]. The depolarizer is a paste of mercurous sulfate. When the electrolyte solution is saturated, the voltage of the cell is very reproducible; hence, in 1911, it was adopted as an international standard for voltage. | |||
A battery is a set of galvanic cells that are connected [[Parallel_circuits#Parallel_circuits|in parallel]]. For instance, a [[lead–acid battery]] has galvanic cells with the anodes composed of lead and cathodes composed of lead dioxide. | |||
==Cell voltage== | |||
The standard electrical potential of a cell can be determined by use of a [[Table of standard electrode potentials|standard potential table]] for the two [[half cell]]s involved. The first step is to identify the two metals reacting in the cell. Then one looks up the [[standard electrode potential]], | |||
''E''<sup>0</sup>, in [[volt]]s, for each of the two [[half-reaction|half reactions]]. The standard potential for the cell is equal to the more positive ''E''<sup>0</sup> value minus the more negative ''E''<sup>0</sup> value. | |||
For example, in the figure above the solutions are CuSO<sub>4</sub> and ZnSO<sub>4</sub>. Each solution has a corresponding metal strip in it, and a salt bridge or porous disk connecting the two solutions and allowing SO<sub>4</sub><sup>2−</sup> ions to flow freely between the copper and zinc solutions. In order to calculate the standard potential one looks up copper and zinc's half reactions and finds: | |||
:Cu<sup>2+</sup> + 2 {{SubatomicParticle|electron}} {{eqm}} Cu: E<sup>0</sup> = +0.34 V | |||
:Zn<sup>2+</sup> + 2 {{SubatomicParticle|electron}} {{eqm}} Zn: E<sup>0</sup> = −0.76 V | |||
Thus the overall reaction is: | |||
:Cu<sup>2+</sup> + Zn {{eqm}} Cu + Zn<sup>2+</sup> | |||
The standard potential for the reaction is then +0.34 V − (−0.76 V) = 1.10 V. The polarity of the cell is determined as follows. Zinc metal is more strongly reducing than copper metal; equivalently, the standard (reduction) potential for zinc is more negative than that of copper. Thus, zinc metal will lose electrons to copper ions and develop a positive electrical charge. The [[equilibrium constant]], ''K'', for the cell is given by | |||
:<math>\ln K= \frac{nFE^0}{RT}</math> | |||
where ''F'' is the [[Faraday constant]], ''R'' is the [[gas constant]] and ''T'' is the temperature in [[kelvin]]s. For the Daniell cell ''K'' is approximately equal to 1.5×10<sup>37</sup>. Thus, at equilibrium, a few electrons are transferred, enough to cause the electrodes to be charged.<ref>{{cite book|last=Atkins|first=P|coauthors=de Paula|others=J.|title=Physical Chemistry|publisher=Oxford University Press|year=2006|edition=8th.|isbn=978-0-19-870072-2}} Chapter 7, sections on "Equilibrium electrochemistry"</ref> | |||
Actual half-cell potentials must be calculated by using the [[Nernst equation]] as the solutes are unlikely to be in their standard states, | |||
:<math>E_{\text{half-cell}} = E^0 - \frac{RT}{nF}\ln_e Q </math> | |||
where ''Q'' is the [[reaction quotient]]. This simplifies to | |||
:<math>E_{\text{half-cell}} = E^0 - 2.303 \frac{RT}{nF} \log_{10} \{ M^{n+}\}</math> | |||
where {''M''<sup>''n''+</sup>} is the [[Activity (chemistry)|activity]] of the metal ion in solution. The metal electrode is in its standard state so by definition has unit activity. In practice concentration is used in place of activity. The potential of the whole cell is obtained by combining the potentials for the two half-cells, so it depends on the concentrations of both dissolved metal ions. | |||
The value of 2.303''R''/''F'' is 0.19845×10<sup>−3</sup> V/K, so at 25 °C (298.15 K) the half-cell potential will change by <math>{0.05918 V}/{n}</math> if the concentration of a metal ion is increased or decreased by a factor of 10. | |||
:<math>E_{\text{half-cell}}= E^0 - \frac{0.05918 V}{n} \log_{10} [ M^{n+}]</math> | |||
These calculations are based on the assumption that all chemical reactions are in equilibrium. When a current flows in the circuit, equilibrium conditions are not achieved and the cell potential will usually be reduced by various mechanisms, such as the development of [[overpotential]]s.<ref>{{cite book|last=Atkins|first=P|coauthors=de Paula|others=J.|title=Physical Chemistry|publisher=Oxford University Press|year=2006|edition=8th.|isbn=978-0-19-870072-2}} Section 25.12 "Working Galvanic cells"</ref> Also, since chemical reactions occur when the cell is producing power, the electrolyte concentrations change and the cell voltage is reduced. A consequence of the temperature dependency of standard potentials is that the voltage produced by a galvanic cell is also temperature dependent. | |||
==Galvanic corrosion== | |||
{{Main|Galvanic corrosion}} | |||
Galvanic corrosion is a process that degrades metals [[electrochemical]]ly. | |||
This [[corrosion]] occurs when two dissimilar metals are placed in contact with each other in the presence of an [[electrolyte]], such as salt water, forming a galvanic cell. A cell can also be formed if the same metal is exposed to two different concentrations of electrolyte. The resulting electrochemical potential then develops an electric current that electrolytically dissolves the less noble material. | |||
==Cell types== | |||
* [[Concentration cell]] | |||
* [[Electrolytic cell]] | |||
* [[Electrochemical cell]] | |||
* [[Lemon battery]] | |||
==See also== | |||
{{Div col}} | |||
* [[resting potential|Biological cell voltage]] | |||
* [[Bio-nano generator]] | |||
* [[Desulfation]] | |||
* [[Electrode potential]] | |||
* [[Electrosynthesis]] | |||
* [[Isotope electrochemistry]] | |||
* [[Bioelectrochemical reactor]] | |||
* [[Enzymatic biofuel cell]] | |||
* [[Electrohydrogenesis]] | |||
* [[Electrochemical engineering]] | |||
* [[Galvanic series]] | |||
* [[Sacrificial anode]] | |||
* [[Volt]] | |||
{{Div col end}} | |||
==References== | |||
{{Reflist}} | |||
*Clarke, Edwin; Jacyna, L. S. [http://books.google.co.uk/books?id=38Sjkp-JlPcC&pg=PA198#v=onepage&q&f=true ''Nineteenth-Century Origins of Neuroscientific Concepts''], University of California Press, 1992 ISBN 0-520-07879-9. | |||
*Kipnis, Nahum [http://ppp.unipv.it/Collana/Pages/Libri/Saggi/Nuova%20Voltiana5_PDF/p__143-162.pdf "Changing a theory: the case of Volta's contact electricity"], ''Nuova Voltiana'', '''vol.5''' (2003), pp. 143–162, Università degli studi di Pavia, 2003 ISBN 88-203-3273-6. | |||
==External links== | |||
* ''[http://web.archive.org/web/20080205130208/http://www.sonoma.edu/users/b/brooks/115b/galvanic.html Galvanic (Voltaic) Cells and Electrode Potential]''. Chemistry 115B, Sonoma.edu. | |||
* ''[http://www.woodrow.org/teachers/chemistry/institutes/1986/exp28.html Making and testing a simple galvanic cell]''. Woodrow Wilson Leadership Program in Chemistry, The Woodrow Wilson National Fellowship Foundation. | |||
* ''[http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/galvan5.swf Galvanic Cell]'' An animation. | |||
* ''[http://www.chem.iastate.edu/group/Greenbowe/sections/projectfolder/flashfiles/electroChem/voltaicCell20.html Interactive animation of Galvanic Cell]''. Chemical Education Research Group, Iowa State University. | |||
* ''[http://www.saskschools.ca/curr_content/chem30_05/6_redox/redox2_2.htm Electrochemical Cells Tutorial Segment]''. Chemistry 30, Saskatchewan Evergreen Curriculum. | |||
{{Galvanic cells}} | |||
[[Category:Galvanic cells| ]] | |||
[[Category:Corrosion]] | |||
Revision as of 21:01, 7 July 2013
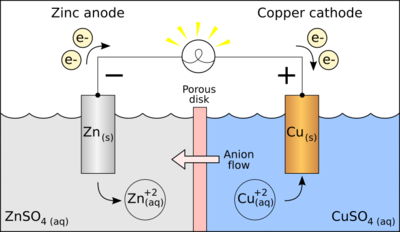
A galvanic cell, or voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane.
Volta was the inventor of the voltaic pile, the first electrical battery. In common usage, the word "battery" has come to include a single galvanic cell, but a battery properly consists of multiple cells.[1]
History
In 1780, Luigi Galvani discovered that when two different metals (e.g., copper and zinc) are connected and then both touched at the same time to two different parts of a nerve of a frog leg, then the leg contracts.[2] He called this "animal electricity". The voltaic pile, invented by Alessandro Volta in the 1800s, consists of a pile of cells similar to the galvanic cell. However, Volta built it entirely out of non-biological material in order to challenge Galvani's (and the later experimenter Leopoldo Nobili) animal electricity theory in favour of his own metal-metal contact electricity theory.[3] Carlo Matteucci in his turn constructed a battery entirely out of biological material in answer to Volta.[4] These discoveries paved the way for electrical batteries; Volta's cell was named an IEEE Milestone in 1999.[5]
It was suggested by Wilhelm König in 1940 that the object known as the Baghdad battery might represent galvanic cell technology from ancient Parthia. Replicas filled with citric acid or grape juice have been shown to produce a voltage. However, it is far from certain that this was its purpose—other scholars have pointed out that it is very similar to vessels known to have been used for storing parchment scrolls.[6]
Description
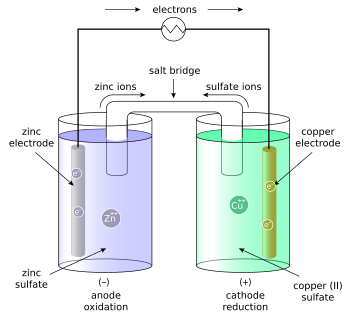
In its simplest form, a half-cell consists of a solid metal (called an electrode) that is submerged in a solution; the solution contains cations of the electrode metal and anions to balance the charge of the cations. In essence, a half-cell contains a metal in two oxidation states; inside an isolated half-cell, there is an oxidation-reduction (redox) reaction that is in chemical equilibrium, a condition written symbolically as follows (here, "M" represents a metal cation, an atom that has a charge imbalance due to the loss of "n" electrons):
- Mn+ (oxidized species) + ne- Template:Eqm M (reduced species)
A galvanic cell consists of two half-cells, such that the electrode of one half-cell is composed of metal A, and the electrode of the other half-cell is composed of metal B; the redox reactions for the two separate half-cells are thus:
- An+ + ne- Template:Eqm A
- Bm+ + me- Template:Eqm B
In general, then, these two metals can react with each other:
- m A + n Bm+ Template:Eqm n B + m An+
In other words, the metal atoms of one half-cell are able to induce reduction of the metal cations of the other half-cell; conversely stated, the metal cations of one half-cell are able to oxidize the metal atoms of the other half-cell. When metal B has a greater electronegativity than metal A, then metal B tends to steal electrons from metal A (that is, metal B tends to oxidize metal A), thus favoring one direction of the reaction:
This reaction between the metals can be controlled in a way that allows for doing useful work:
- The electrodes are connected with a metal wire in order to conduct the electrons that participate in the reaction.
- In one half-cell, dissolved metal-B cations combine with the free electrons that are available at the interface between the solution and the metal-B electrode; these cations are thereby neutralized, causing them to precipitate from solution as deposits on the metal-B electrode, a process known as plating.
- This reduction reaction causes the free electrons throughout the metal-B electrode, the wire, and the metal-A electrode to be pulled into the metal-B electrode. Consequently, electrons are wrestled away from some of the atoms of the metal-A electrode, as though the metal-B cations were reacting directly with them; those metal-A atoms become cations that dissolve into the surrounding solution.
- As this reaction continues, the half-cell with the metal-A electrode develops a positively charged solution (because the metal-A cations dissolve into it), while the other half-cell develops a negatively charged solution (because the metal-B cations precipitate out of it, leaving behind the anions); unabated, this imbalance in charge would stop the reaction.
- The solutions are connected by a salt bridge or a porous plate in order to conduct the ions (both the metal-A cations from one solution, and the anions from the other solution), which balances the charges of the solutions and thereby allows the reaction between metal A and metal B to continue without opposition.
By definition:
- The anode is the electrode where oxidation (loss of electrons) takes place; the anode attracts anions. The metal-A electrode is the anode.
- The cathode is the electrode where reduction (gain of electrons) takes place; the cathode attracts cations. The metal-B electrode is the cathode.
Copper readily oxidizes zinc; for the Daniell cell depicted in the figure, the anode is zinc and the cathode is copper, and the anions in the solutions are sulfates of the respective metals. When an electrically conducting device connects the electrodes, the electrochemical reaction is:
- Zn + Template:Chem → Template:Chem+ Cu
The zinc electrode is dissolved and copper is deposited on the copper electrode.
Galvanic cells are typically used as a source of electrical power. By their nature, they produce direct current. The Weston cell has an anode composed of cadmium mercury amalgam, and a cathode composed of pure mercury. The electrolyte is a (saturated) solution of cadmium sulfate. The depolarizer is a paste of mercurous sulfate. When the electrolyte solution is saturated, the voltage of the cell is very reproducible; hence, in 1911, it was adopted as an international standard for voltage.
A battery is a set of galvanic cells that are connected in parallel. For instance, a lead–acid battery has galvanic cells with the anodes composed of lead and cathodes composed of lead dioxide.
Cell voltage
The standard electrical potential of a cell can be determined by use of a standard potential table for the two half cells involved. The first step is to identify the two metals reacting in the cell. Then one looks up the standard electrode potential, E0, in volts, for each of the two half reactions. The standard potential for the cell is equal to the more positive E0 value minus the more negative E0 value.
For example, in the figure above the solutions are CuSO4 and ZnSO4. Each solution has a corresponding metal strip in it, and a salt bridge or porous disk connecting the two solutions and allowing SO42− ions to flow freely between the copper and zinc solutions. In order to calculate the standard potential one looks up copper and zinc's half reactions and finds:
- Cu2+ + 2 Template:SubatomicParticle Template:Eqm Cu: E0 = +0.34 V
- Zn2+ + 2 Template:SubatomicParticle Template:Eqm Zn: E0 = −0.76 V
Thus the overall reaction is:
- Cu2+ + Zn Template:Eqm Cu + Zn2+
The standard potential for the reaction is then +0.34 V − (−0.76 V) = 1.10 V. The polarity of the cell is determined as follows. Zinc metal is more strongly reducing than copper metal; equivalently, the standard (reduction) potential for zinc is more negative than that of copper. Thus, zinc metal will lose electrons to copper ions and develop a positive electrical charge. The equilibrium constant, K, for the cell is given by
where F is the Faraday constant, R is the gas constant and T is the temperature in kelvins. For the Daniell cell K is approximately equal to 1.5×1037. Thus, at equilibrium, a few electrons are transferred, enough to cause the electrodes to be charged.[7]
Actual half-cell potentials must be calculated by using the Nernst equation as the solutes are unlikely to be in their standard states,
where Q is the reaction quotient. This simplifies to
where {Mn+} is the activity of the metal ion in solution. The metal electrode is in its standard state so by definition has unit activity. In practice concentration is used in place of activity. The potential of the whole cell is obtained by combining the potentials for the two half-cells, so it depends on the concentrations of both dissolved metal ions.
The value of 2.303R/F is 0.19845×10−3 V/K, so at 25 °C (298.15 K) the half-cell potential will change by if the concentration of a metal ion is increased or decreased by a factor of 10.
These calculations are based on the assumption that all chemical reactions are in equilibrium. When a current flows in the circuit, equilibrium conditions are not achieved and the cell potential will usually be reduced by various mechanisms, such as the development of overpotentials.[8] Also, since chemical reactions occur when the cell is producing power, the electrolyte concentrations change and the cell voltage is reduced. A consequence of the temperature dependency of standard potentials is that the voltage produced by a galvanic cell is also temperature dependent.
Galvanic corrosion
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church. Galvanic corrosion is a process that degrades metals electrochemically. This corrosion occurs when two dissimilar metals are placed in contact with each other in the presence of an electrolyte, such as salt water, forming a galvanic cell. A cell can also be formed if the same metal is exposed to two different concentrations of electrolyte. The resulting electrochemical potential then develops an electric current that electrolytically dissolves the less noble material.
Cell types
See also
Organisational Psychologist Alfonzo Lester from Timmins, enjoys pinochle, property developers in new launch singapore property and textiles. Gets motivation through travel and just spent 7 days at Alejandro de Humboldt National Park.
- Biological cell voltage
- Bio-nano generator
- Desulfation
- Electrode potential
- Electrosynthesis
- Isotope electrochemistry
- Bioelectrochemical reactor
- Enzymatic biofuel cell
- Electrohydrogenesis
- Electrochemical engineering
- Galvanic series
- Sacrificial anode
- Volt
42 year-old Environmental Consultant Merle Eure from Hudson, really loves snowboarding, property developers in new launch ec singapore and cosplay. Maintains a trip blog and has lots to write about after visiting Chhatrapati Shivaji Terminus (formerly Victoria Terminus).
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
- Clarke, Edwin; Jacyna, L. S. Nineteenth-Century Origins of Neuroscientific Concepts, University of California Press, 1992 ISBN 0-520-07879-9.
- Kipnis, Nahum "Changing a theory: the case of Volta's contact electricity", Nuova Voltiana, vol.5 (2003), pp. 143–162, Università degli studi di Pavia, 2003 ISBN 88-203-3273-6.
External links
- Galvanic (Voltaic) Cells and Electrode Potential. Chemistry 115B, Sonoma.edu.
- Making and testing a simple galvanic cell. Woodrow Wilson Leadership Program in Chemistry, The Woodrow Wilson National Fellowship Foundation.
- Galvanic Cell An animation.
- Interactive animation of Galvanic Cell. Chemical Education Research Group, Iowa State University.
- Electrochemical Cells Tutorial Segment. Chemistry 30, Saskatchewan Evergreen Curriculum.
- ↑ "battery" (def. 4b), Merriam-Webster Online Dictionary (2008). Retrieved 6 August 2008.
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Kipnis, pages 144-146
- ↑ Clarke & Jacyna, page 199
- ↑ Template:Cite web
- ↑ Brian Haughton, Hidden History: Lost Civilizations, Secret Knowledge, and Ancient Mysteries, pages 129-132, Career Press, 2007 ISBN 1564148971.
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 Chapter 7, sections on "Equilibrium electrochemistry" - ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 Section 25.12 "Working Galvanic cells"





![{\displaystyle E_{\text{half-cell}}=E^{0}-{\frac {0.05918V}{n}}\log _{10}[M^{n+}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2e8c3cad062d227bf93137f8992ab640ba47e98d)